Reservoir type ropivacaine pharmaceutical composition as well as preparation method and application thereof
A technology of ropivacaine and its composition, which is applied in the storage type ropivacaine pharmaceutical composition and the field of preparation of drugs for treating postoperative pain, and can solve gastrointestinal adverse reactions, irritation and systemic adverse reactions, drugs Low effective utilization and other problems, to achieve the effect of improving bioavailability and effective bioavailability, solving clinical treatment and safety needs, and prolonging effective treatment time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Preparation of depot pharmaceutical compositions
[0135] The preparation process of the composition in Table 8: add the prescribed amount of ropivacaine into the drug solvent, stir until dissolved to obtain a drug solution; add the medicinal phospholipids to the corresponding medicinal oils, and shear at high speed to obtain the medicinal phospholipids Completely dissolve; add the drug solution therein, stir evenly, then add the drug effect enhancer, stir to obtain the drug-containing solution, filter and subpackage, fill with nitrogen, seal, and then sterilize with moist heat to obtain a clear and transparent liquid.
[0136] Table 8. Pharmaceutical Composition Prescription
[0137]
[0138] The results show that the compositions prepared after adding an appropriate amount of medicinal phospholipids are all clear and transparent, and the purpose of improving the solubility of the drug in the composition system is achieved.
[0139] The preparation process of the...
experiment example 1
[0153] Experimental Example 1 Stability Investigation
[0154] Place the pharmaceutical composition of Preparation Example 1 to observe its appearance to judge its stability. When there is no visible precipitate, it can be considered that the standing stability of the composition is good.
[0155] Table 13. Stability investigation results of pharmaceutical compositions
[0156]
[0157]
[0158] The results showed that the prepared compositions were all clear and transparent, indicating that they had good storage stability. However, the preparation provided by CN109316602A (i.e. Comparative Example 6) had obvious precipitation after being left for 5 hours, showing poor stability.
[0159] Select part of the composition and put it under the corresponding conditions, and detect the content and the total amount of related substances according to the following method.
[0160] Testing equipment:
[0161] High performance liquid chromatograph 1260, Agilent Technologies Co...
experiment example 2
[0174] Experimental example 2 Acupuncture Determination of Pharmaceutical Compositions
[0175] A 21G needle was used to test the penetrability of the composition, and the test results are shown in Table 15.
[0176] Table 15. Results of Viscosity Measurements for Pharmaceutical Compositions
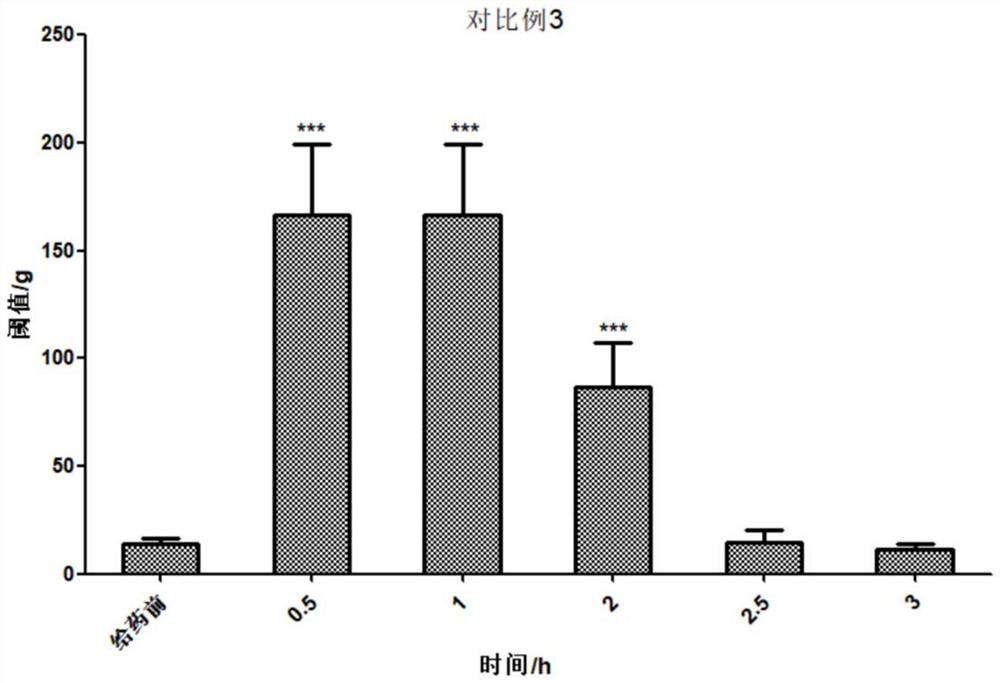
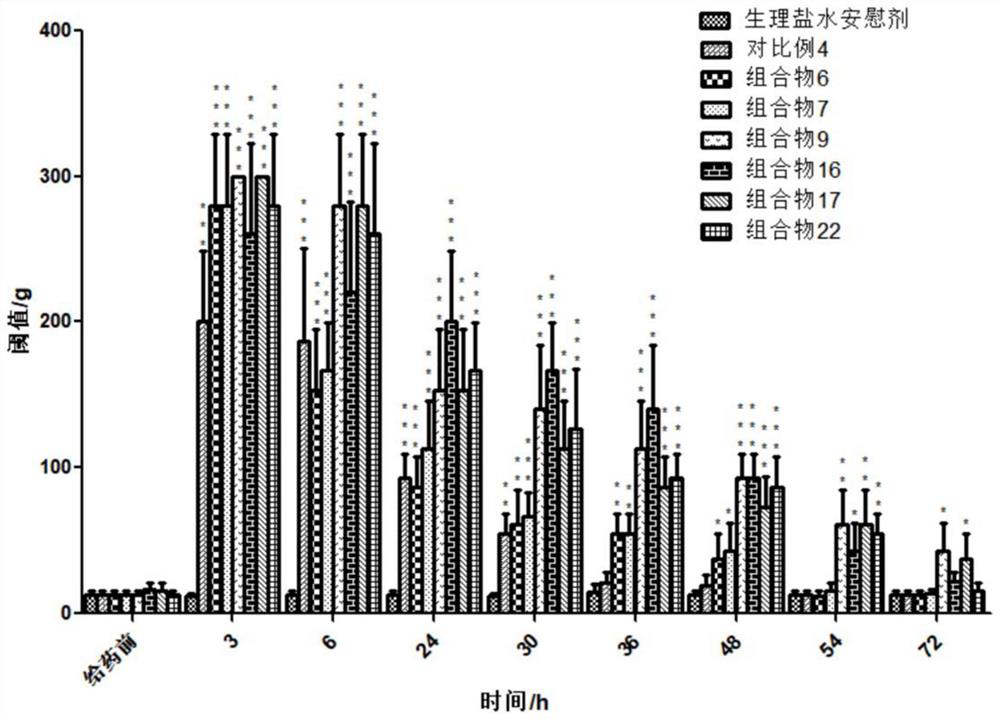
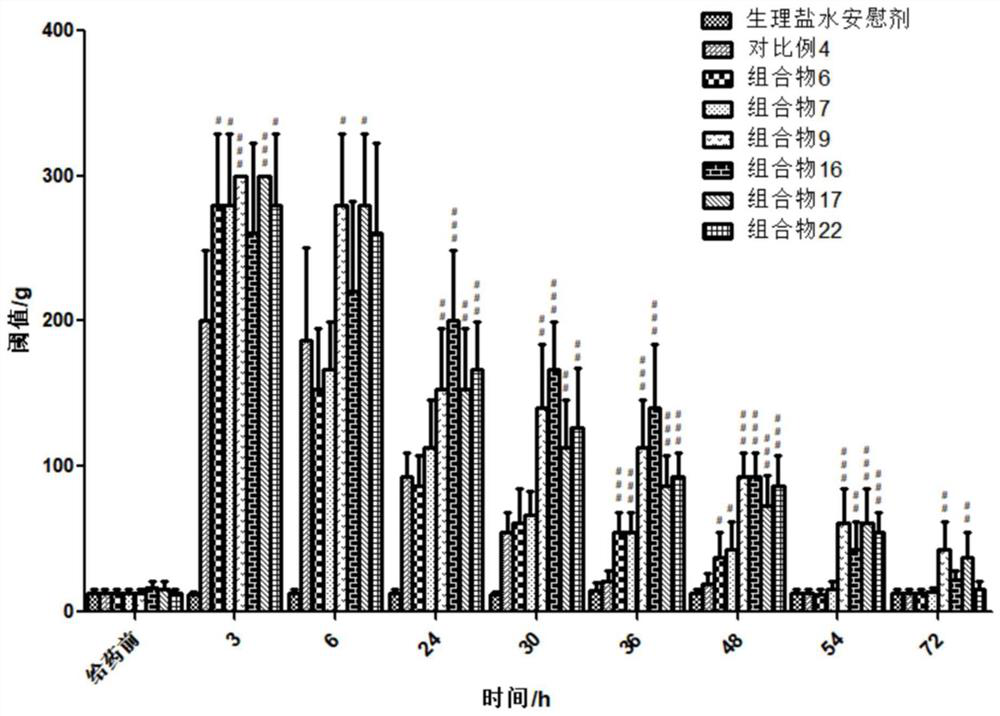
[0177] combination injectable Composition 3 to Composition 25 Moderate viscosity, can pass through 21G needle smoothly Comparative example 4 Difficult injection, high viscosity, poor needle penetration Comparative example 5 Low viscosity, can pass through 21G needle smoothly
[0178] The results show that the composition involved in the present invention has suitable needle passability; while the injection of Comparative Example 4 is difficult and the needle passability is extremely poor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com