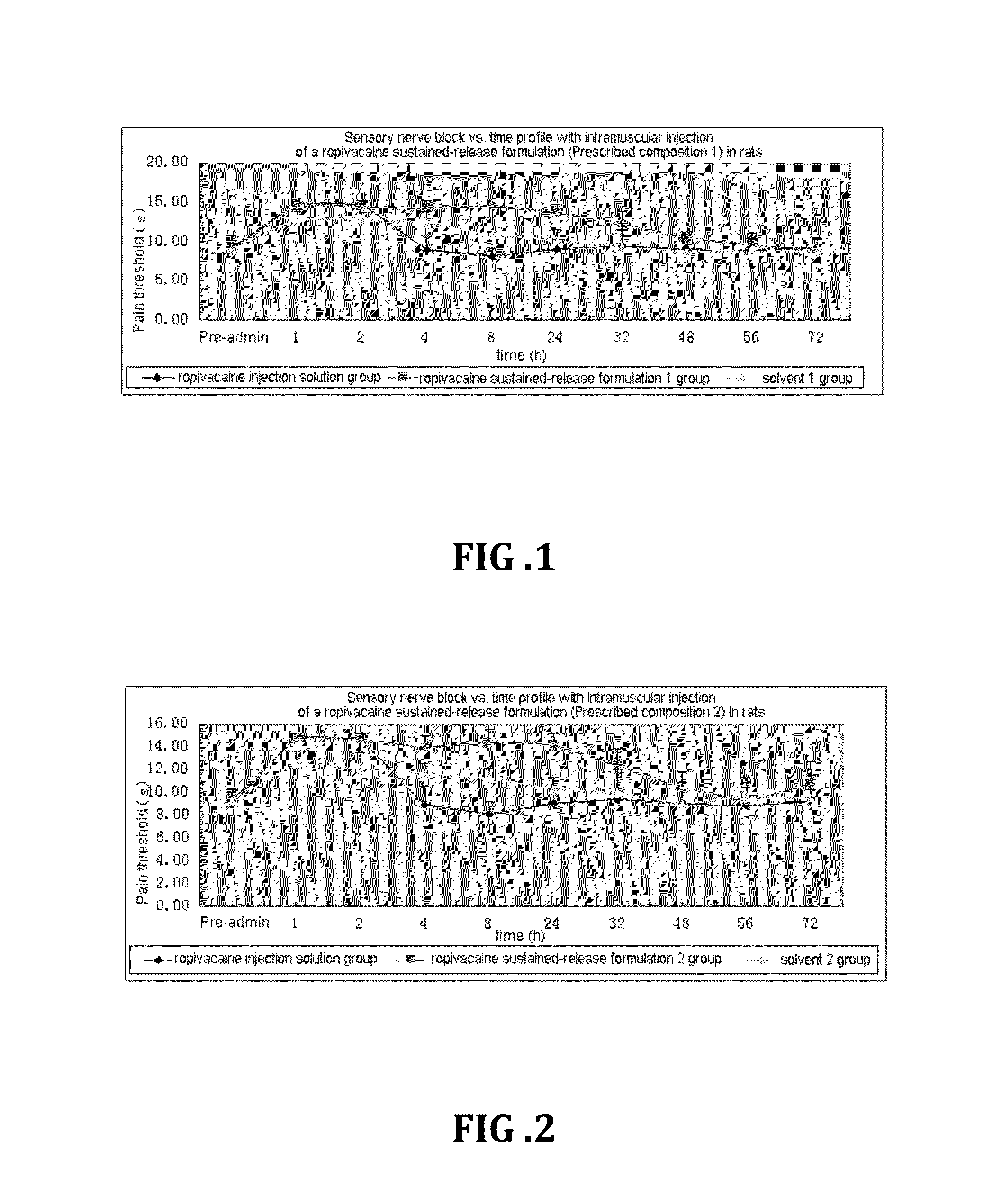
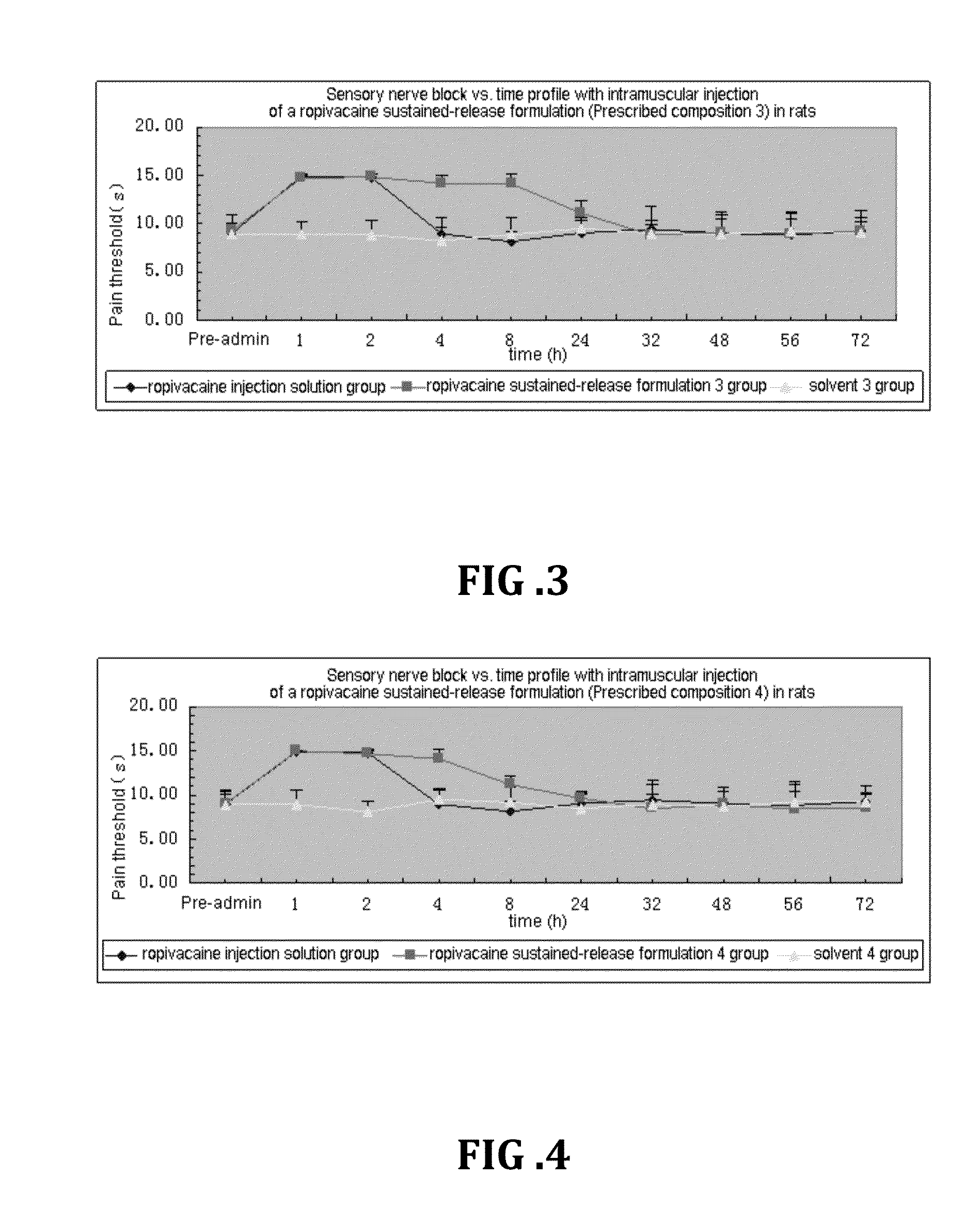
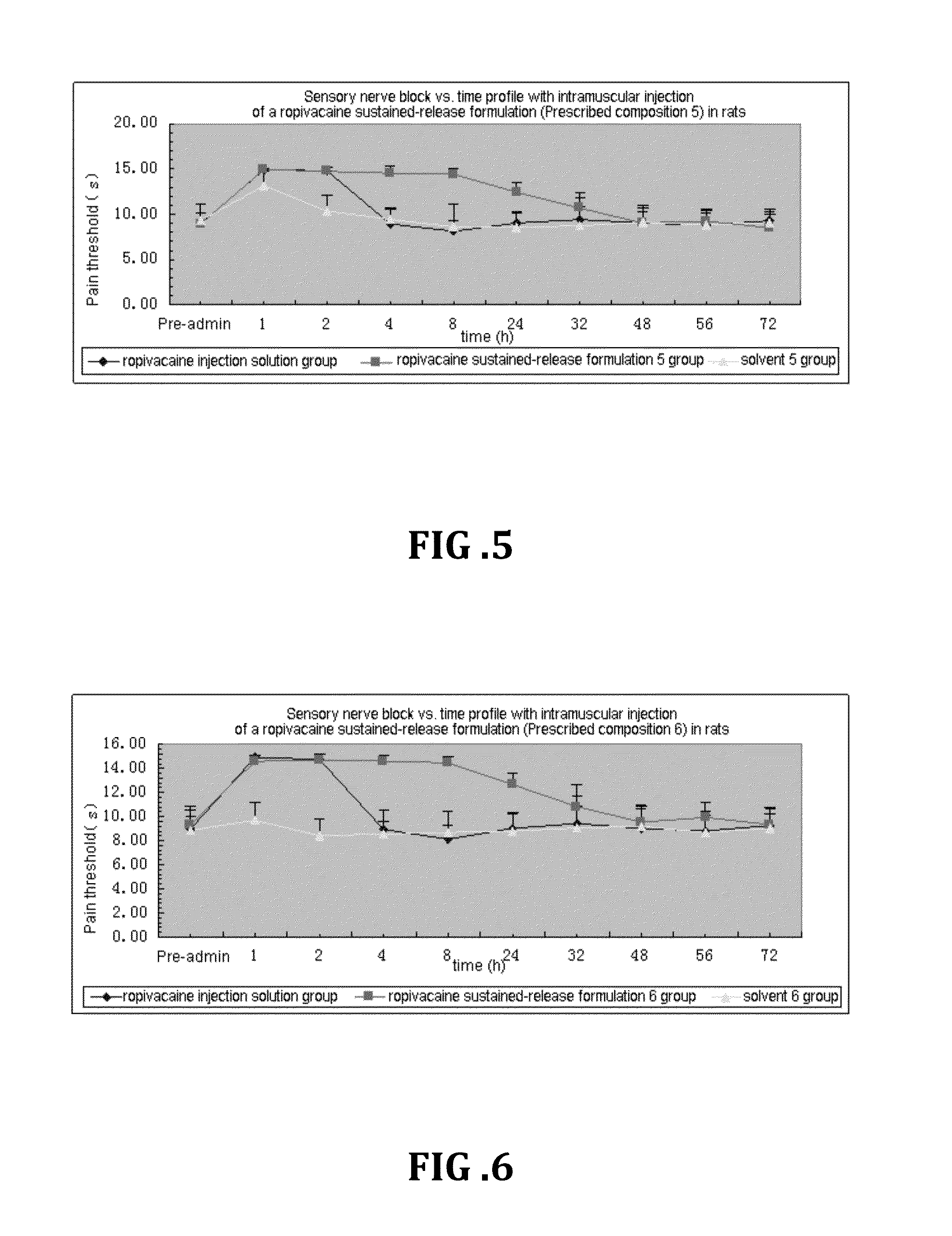
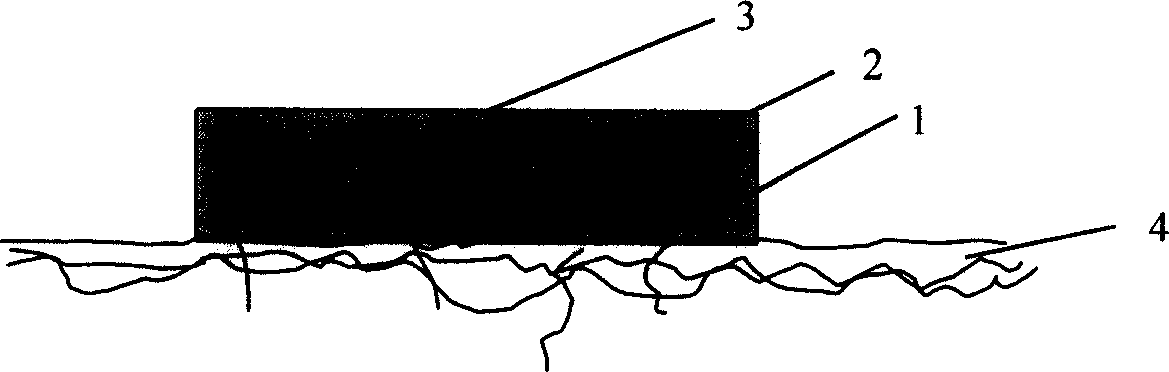
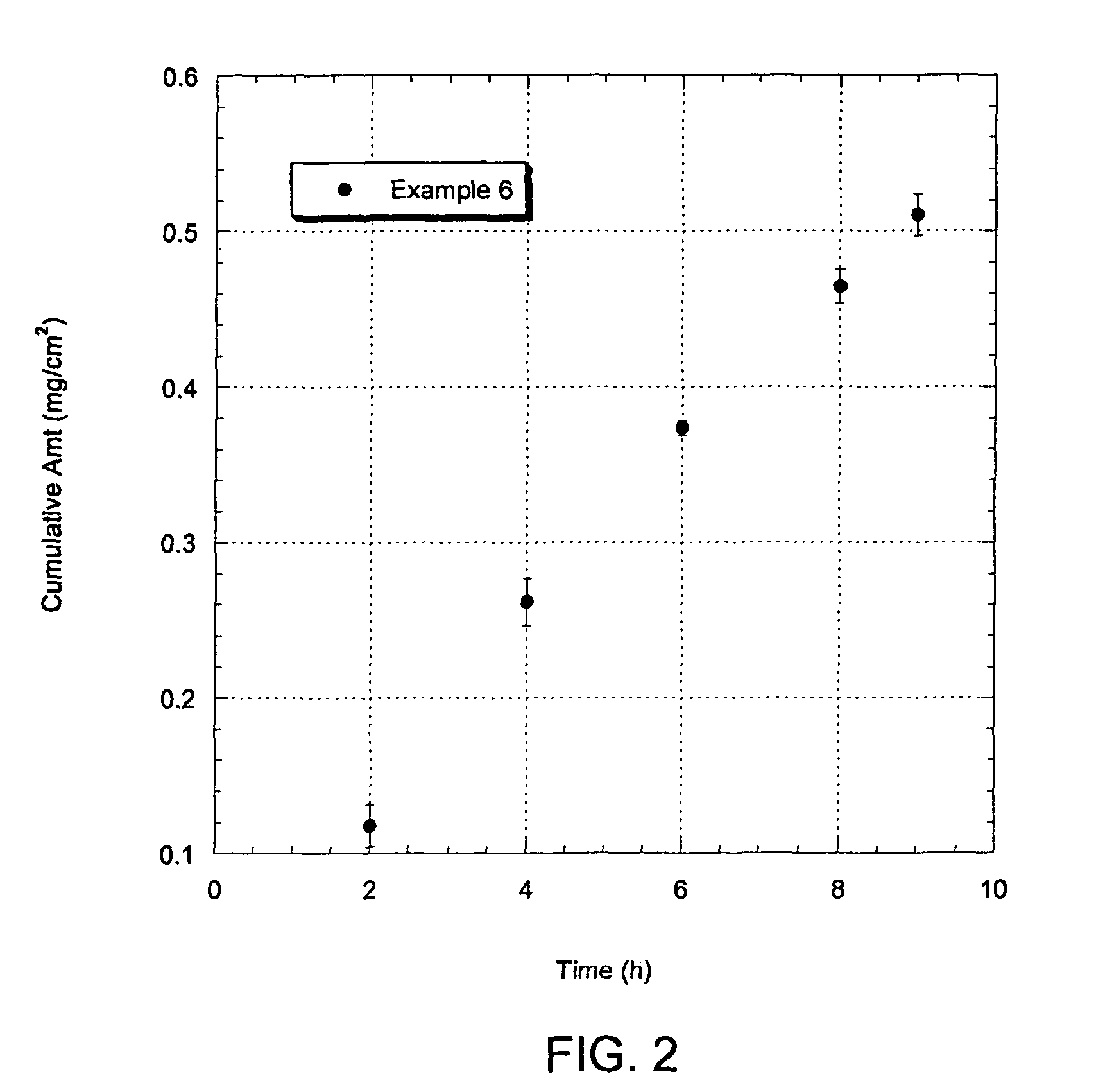
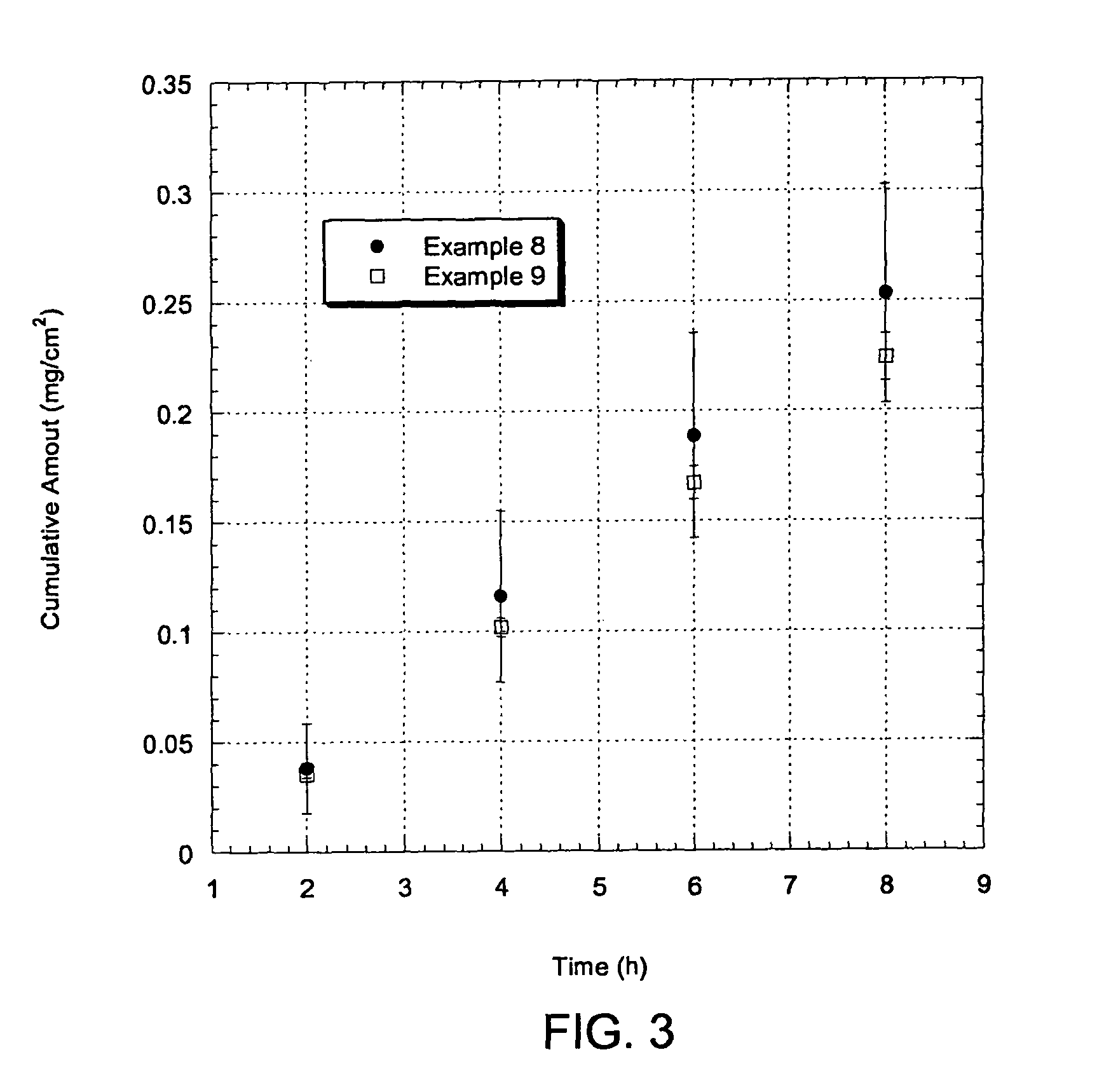
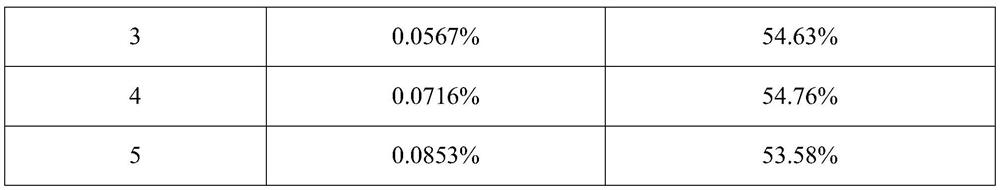
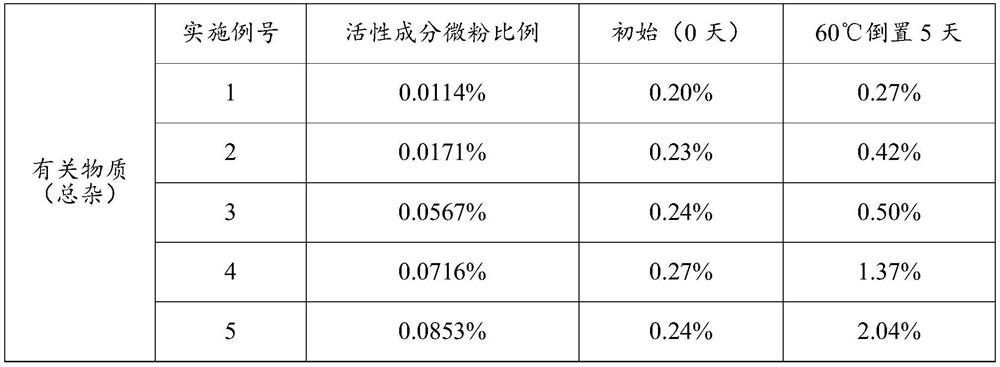
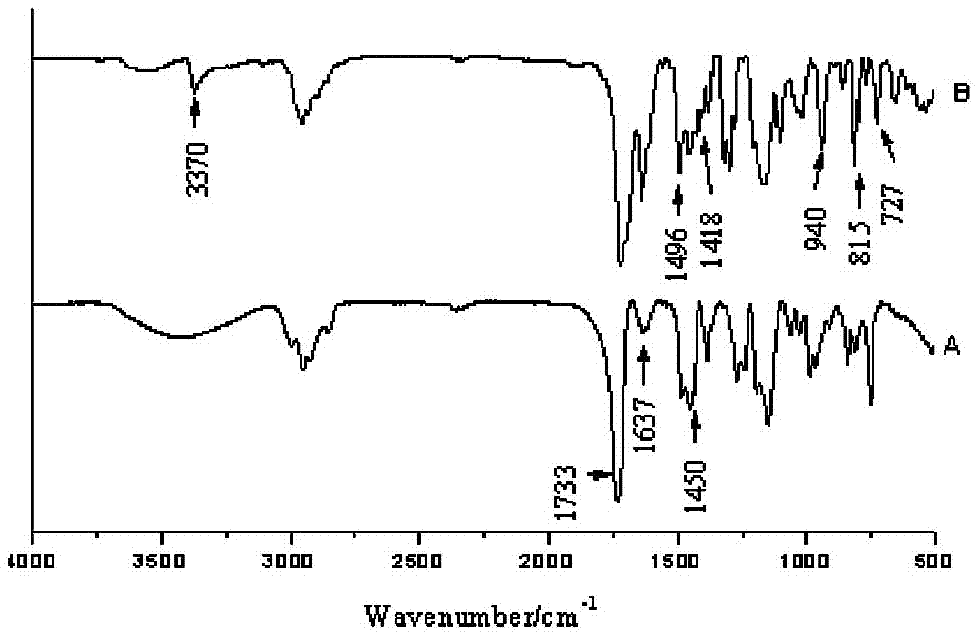
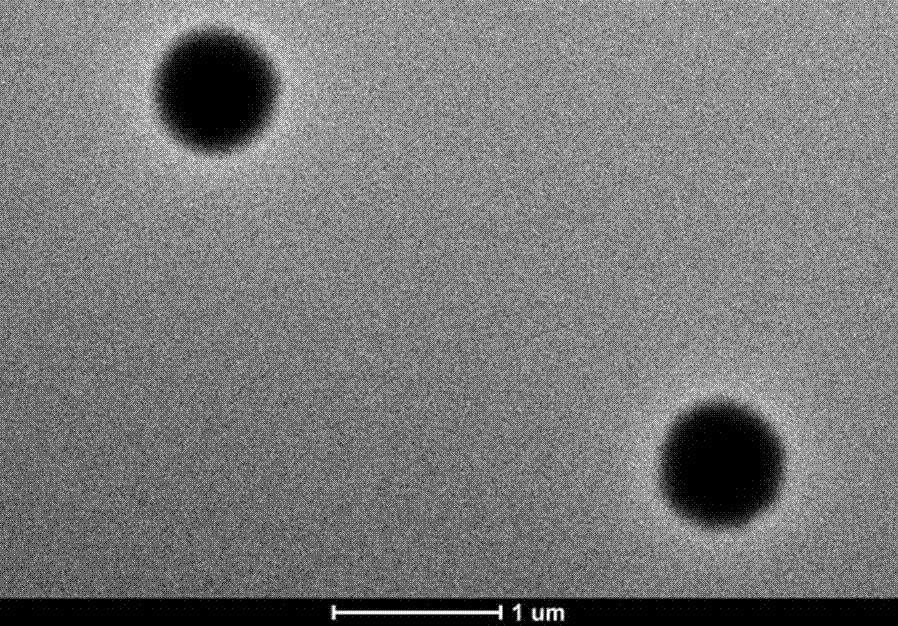
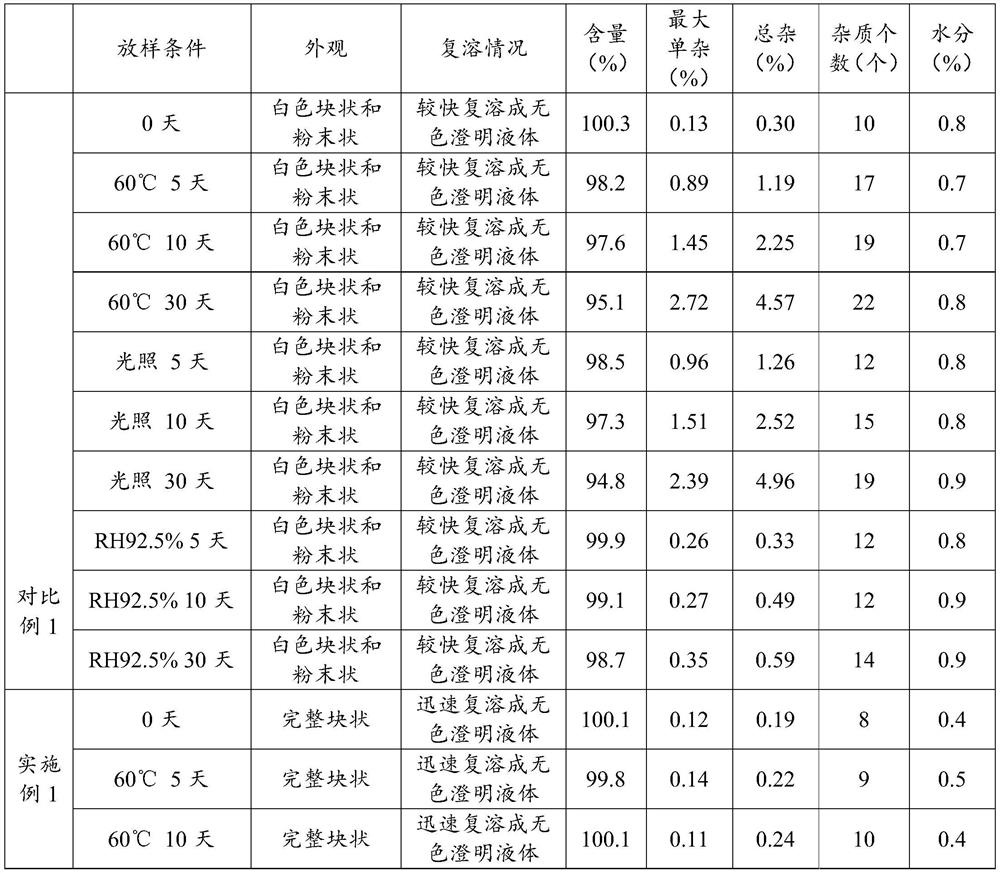
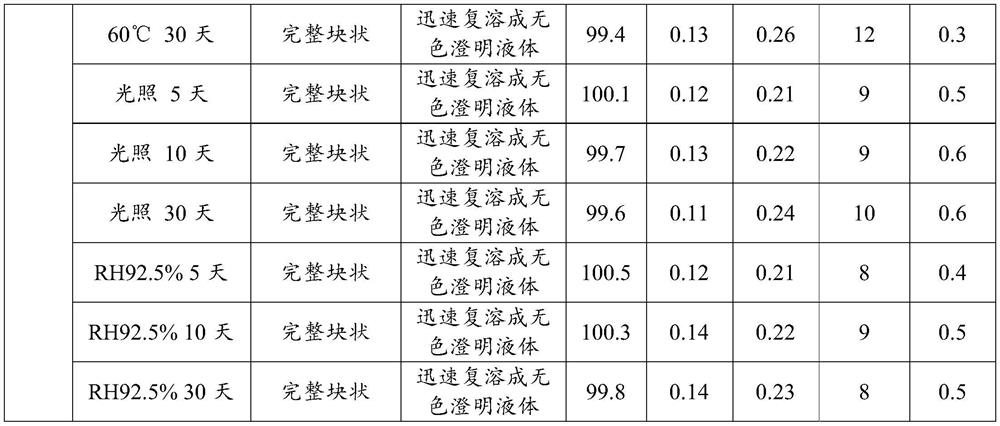
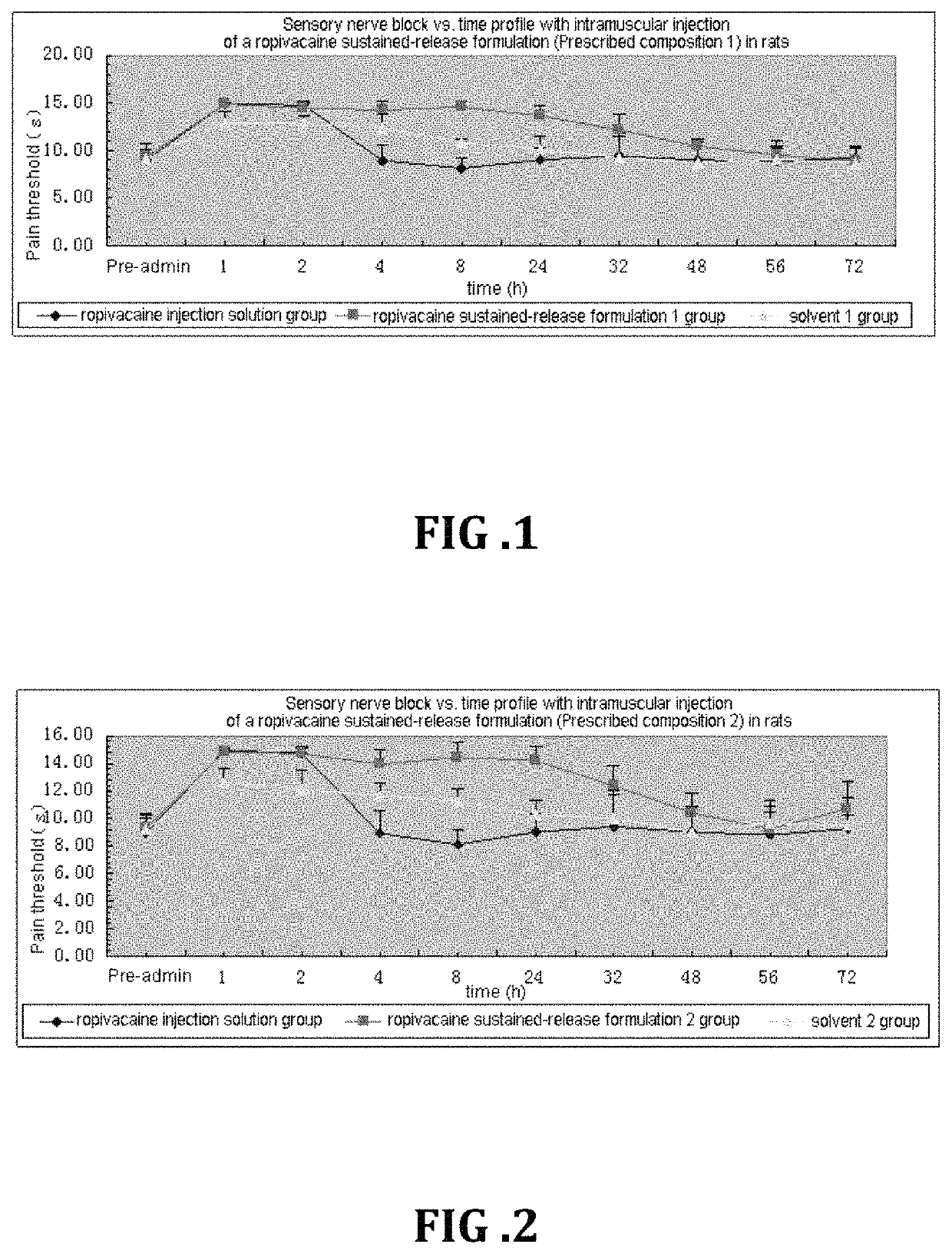
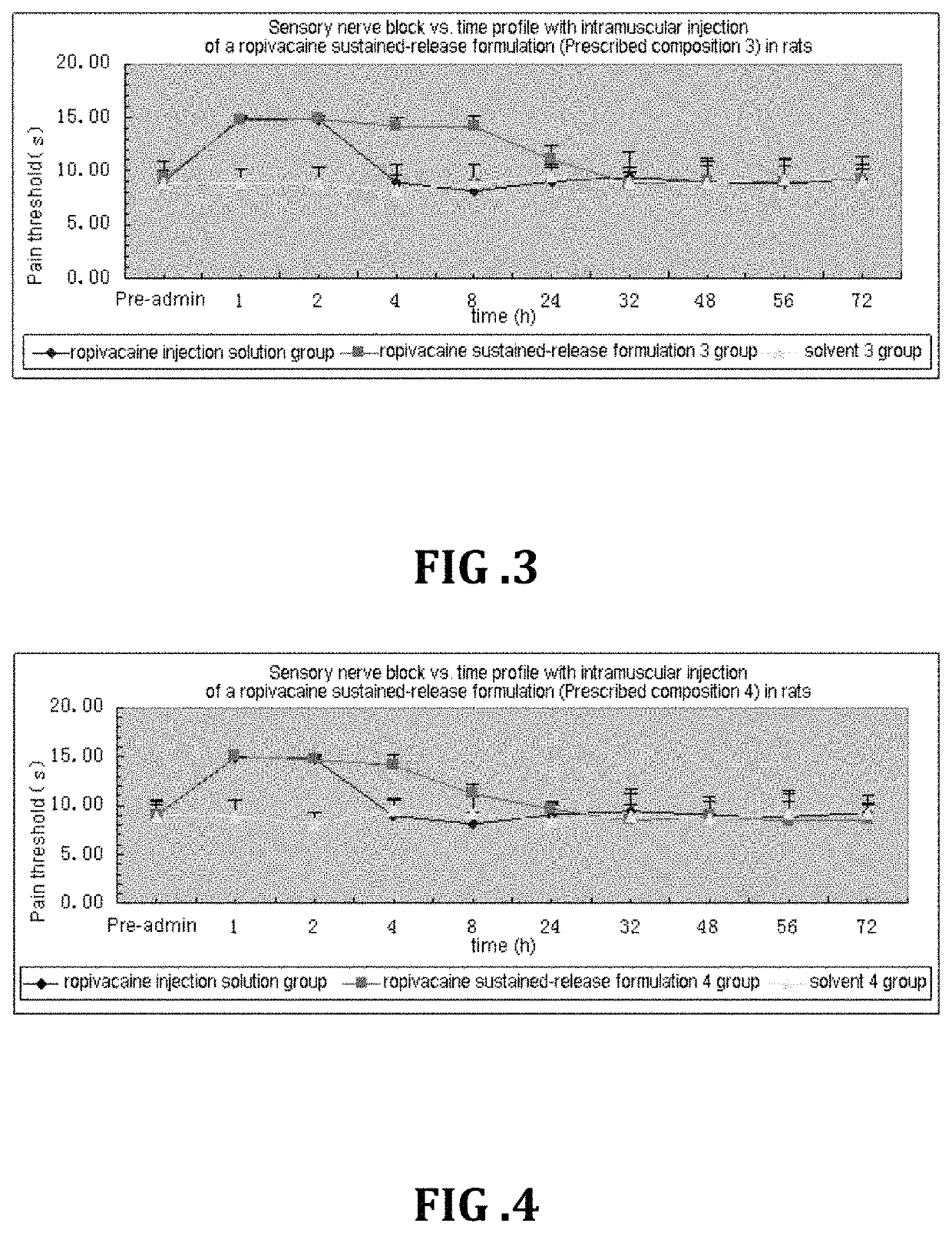
Patents
Literature
42 results about "Pharmaceutical solvent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
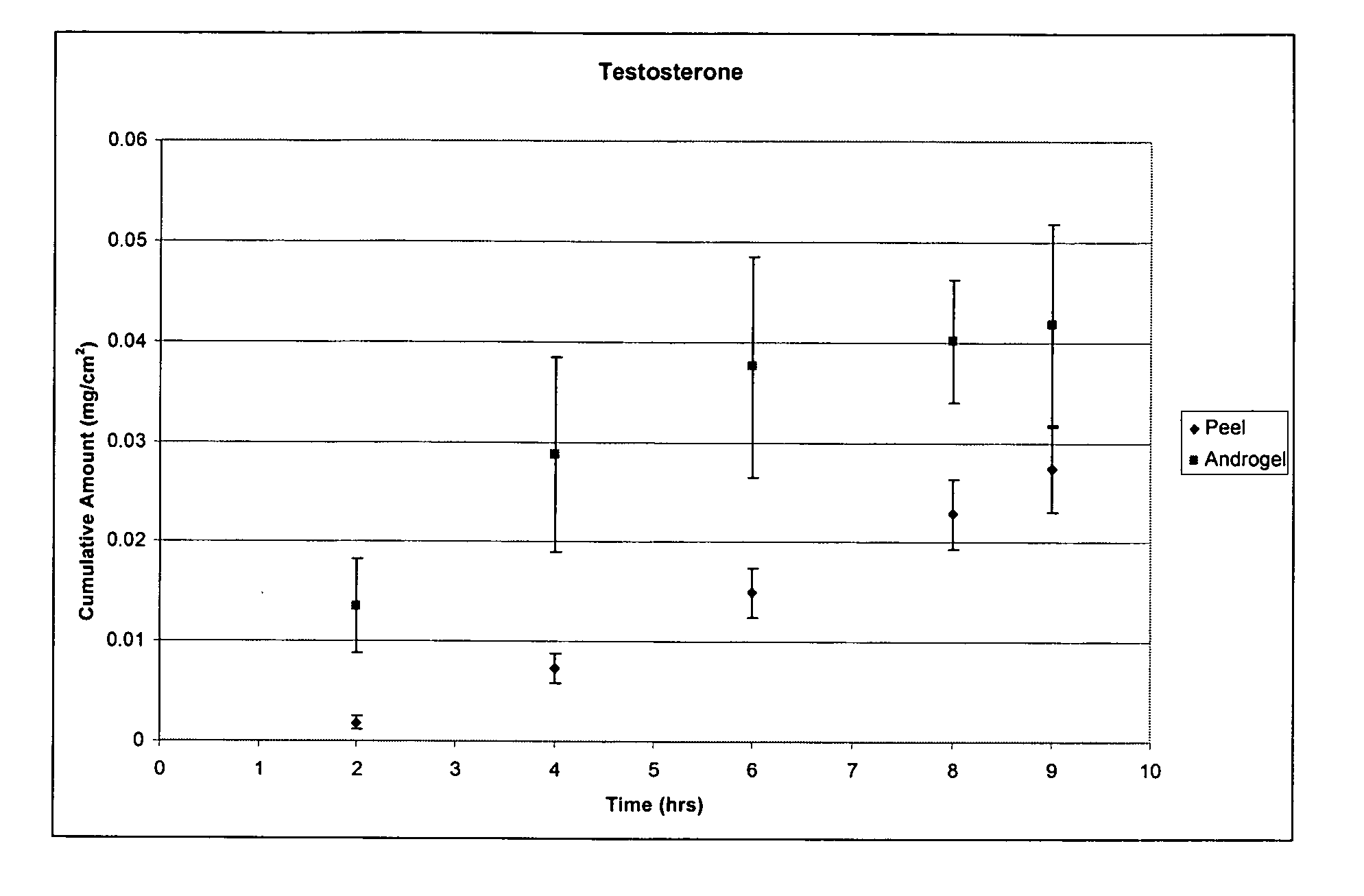
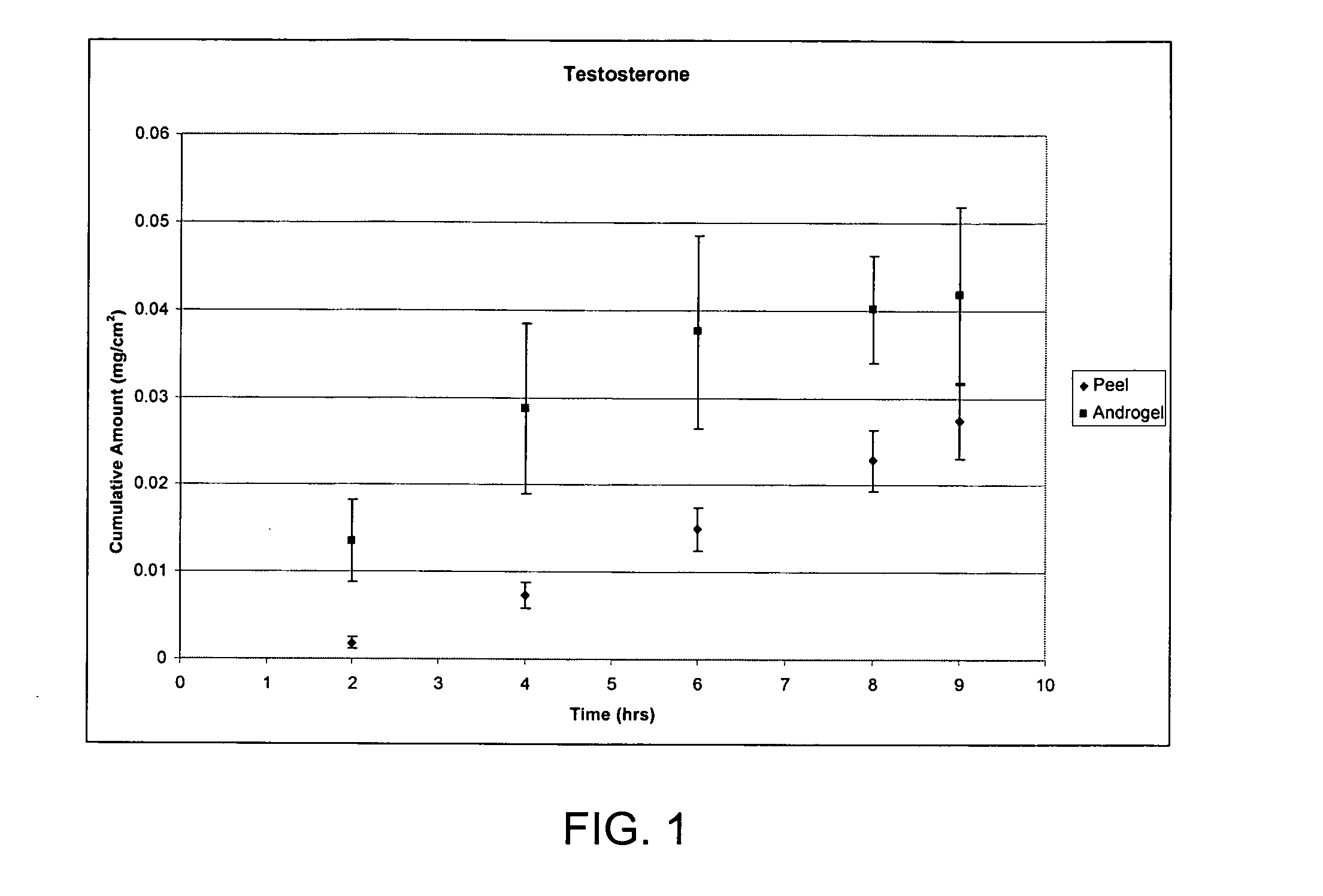
Two or more non-volatile solvent-containing compositions and methods for dermal delivery of drugs
InactiveUS20070196453A1Reduce deliveryEasy to disassembleOrganic active ingredientsMacromolecular non-active ingredientsEvaporationDermatology
The present invention is drawn to adhesive formulations, methods of drug delivery, and solidified layers for dermal delivery of a drug. The formulation can include a drug, a solvent vehicle, and a solidifying agent. The solvent vehicle can have a volatile solvent system including at least one volatile solvent, and a non-volatile solvent system including at least two non-volatile solvents. The formulation can have a viscosity suitable for application to a skin surface prior to evaporation of the volatile solvents system. When applied to the skin, the formulation can form a solidified layer after at least a portion of the volatile solvent system is evaporated.
Owner:NUVO RES
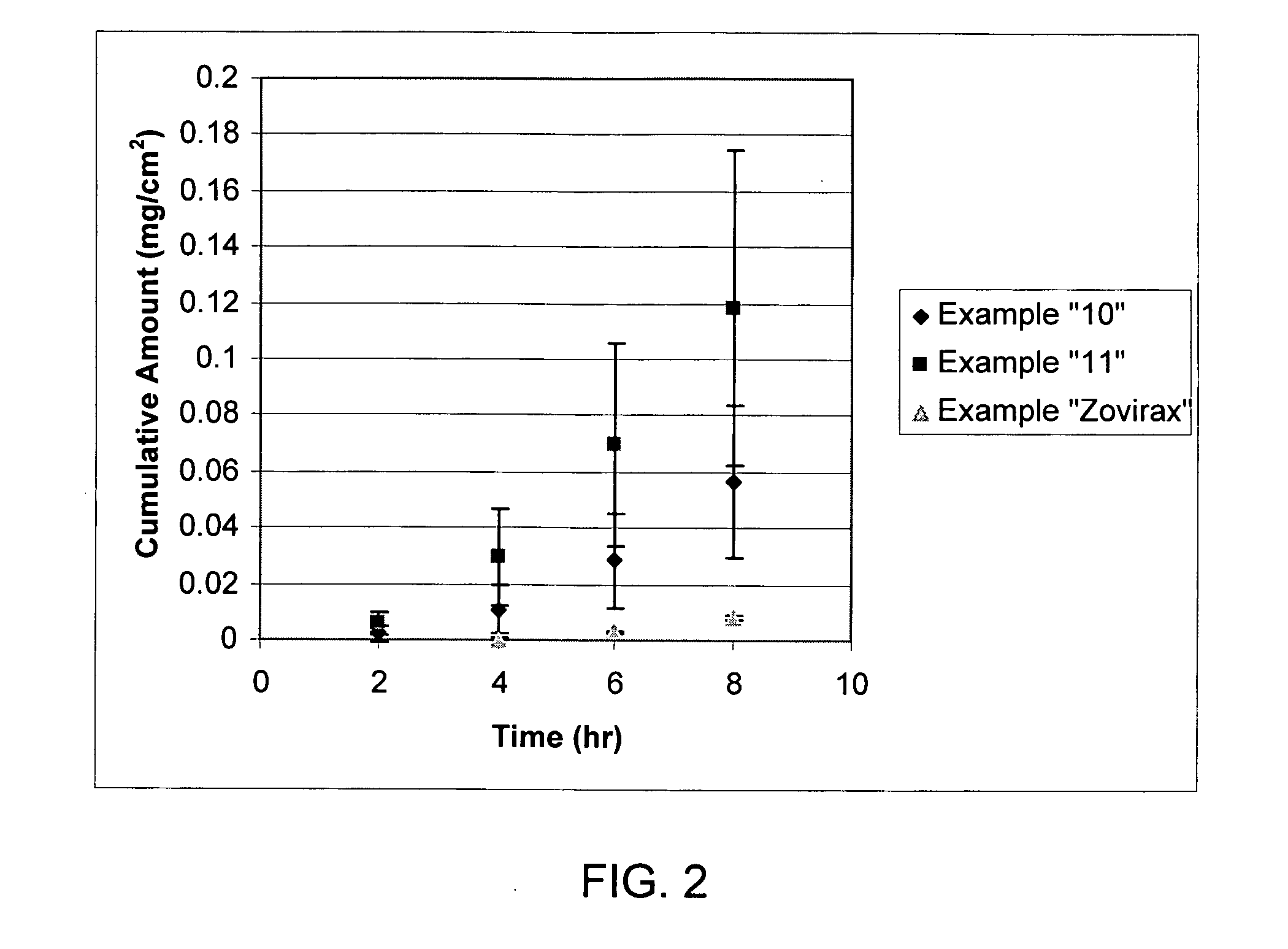
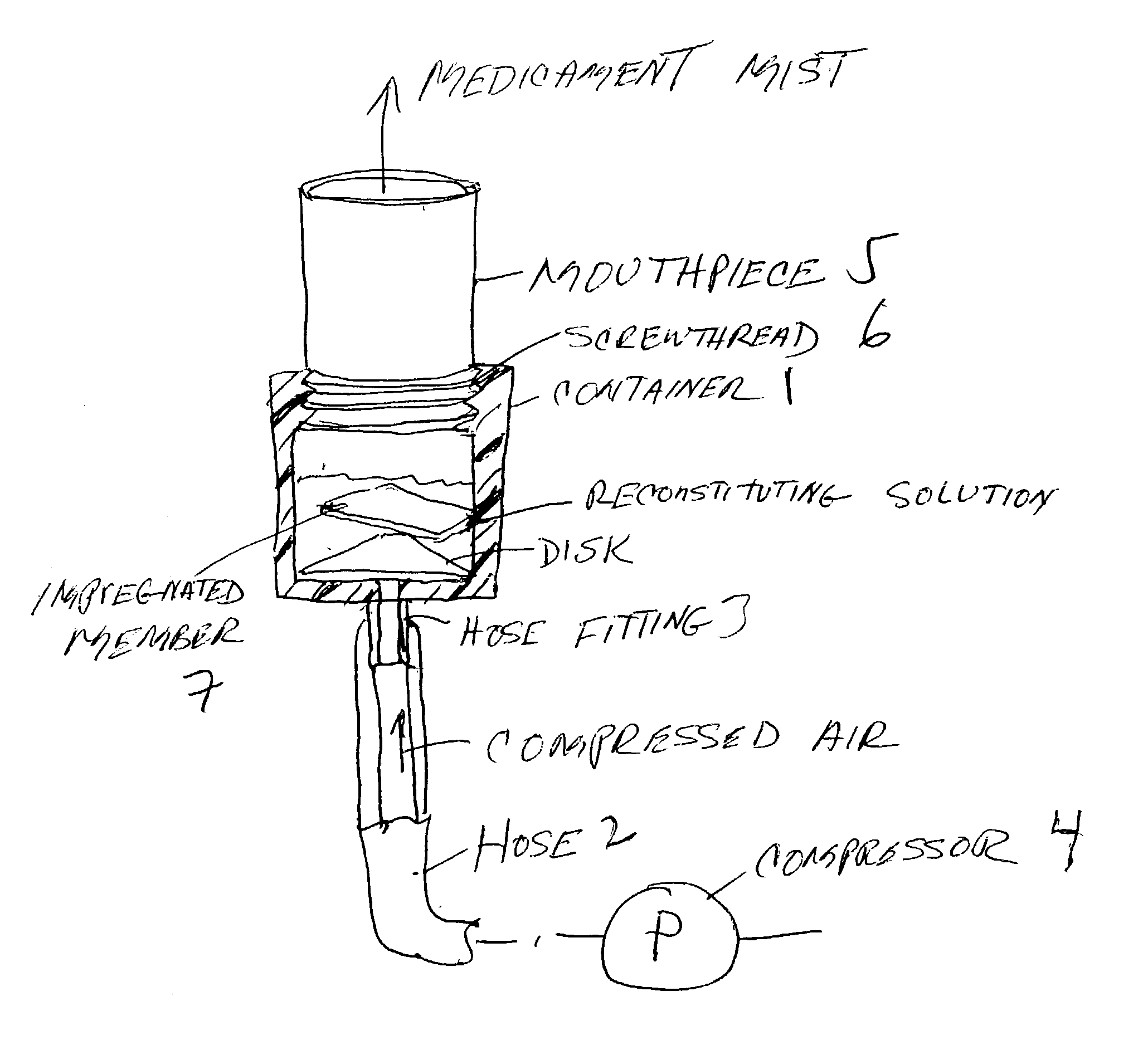
Pharmaceutical delivery system for oral inhalation through nebulization consisting of inert substrate impregnated with substance (S) to be solubilized or suspended prior to use
InactiveUS20040045546A1Good chemical stabilitySolvent evaporates quicklyDispersion deliverySolution deliveryBULK ACTIVE INGREDIENTDelivery system
A pharmaceutical delivery system for oral inhalation is disclosed through nebulization consisting of an inert supporting material impregnated with or deposited with pharmaceutically active ingredient which must be solubilized or suspended in a pharmaceutical solvent to form a solution or suspension prior to administration. Each pharmaceutical delivery unit dosage form comprises one or more therapeutically effective and safe amounts of pharmaceutically active ingredient uniformly impregnated in or deposited on a supporting material which is a natural or synthetic polymer, woven or non-woven fabrics, inert paper, inorganic materials such as foil and combination thereof in a single or multi-layer lamination in a form of a sheet or strip or film or membrane or sponge-like or cup or well. The dosage form of this invention is to be administered to a patient through oral or nasal inhalation using a nebulizer after reconstitution with a reconstituting solvent.
Owner:COLLEGIUM PHARMA INC
Adhesive solid gel-forming formulations for dermal drug delivery
The present invention is drawn to adhesive solid gel-forming formulations, methods of drug delivery, and solidified gel layers for dermal delivery of a drug. The formulation can include a drug, a solvent vehicle, and a gelling agent. The solvent vehicle can include a volatile solvent system having one or more volatile solvent, and a non-volatile solvent system having one or more non-volatile solvent, wherein at least one non-volatile solvent is flux-enabling non-volatile solvent(s) capable of facilitating the delivery of the drug at therapeutically effective rates over a sustained period of time. The formulation can have a viscosity suitable for application to a skin surface prior to evaporation of the volatile solvents system. When applied to the skin, the formulation can form a solidified gel layer after at least a portion of the volatile solvent system is evaporated. The solidified gel layer is can be removed by either peeling or washing using a designated solvent or solvents.
Owner:NUVO RES
Compositions and methods for dermally treating musculoskeletal pain
InactiveUS20070189978A1Suitable viscosityFacilitate transdermal deliveryBiocidePeptide/protein ingredientsJoint painSkin treatments
The present invention is drawn to solidifying formulations for dermal delivery of a drug for treating musculoskeletal pain, inflammation, joint pain, etc. The formulation can include a drug selected from certain drug classes, a solvent vehicle, and a solidifying agent. The solvent vehicle can include a volatile solvent system having one or more volatile solvent, and a non-volatile solvent system having one or more non-volatile solvent, wherein the evaporation of at least some of the volatile solvent converts the formulation on the skin into a solidified layer and the non-volatile solvent system is capable of facilitating the topical delivery of the drug(s) at therapeutically effective rates over a sustained period of time.
Owner:ZARS INC
Solvent system of hardly soluble drug with improved dissolution rate
InactiveUS20090318558A1Good disintegrationPromote dissolutionBiocideAntipyreticActive agentDissolution
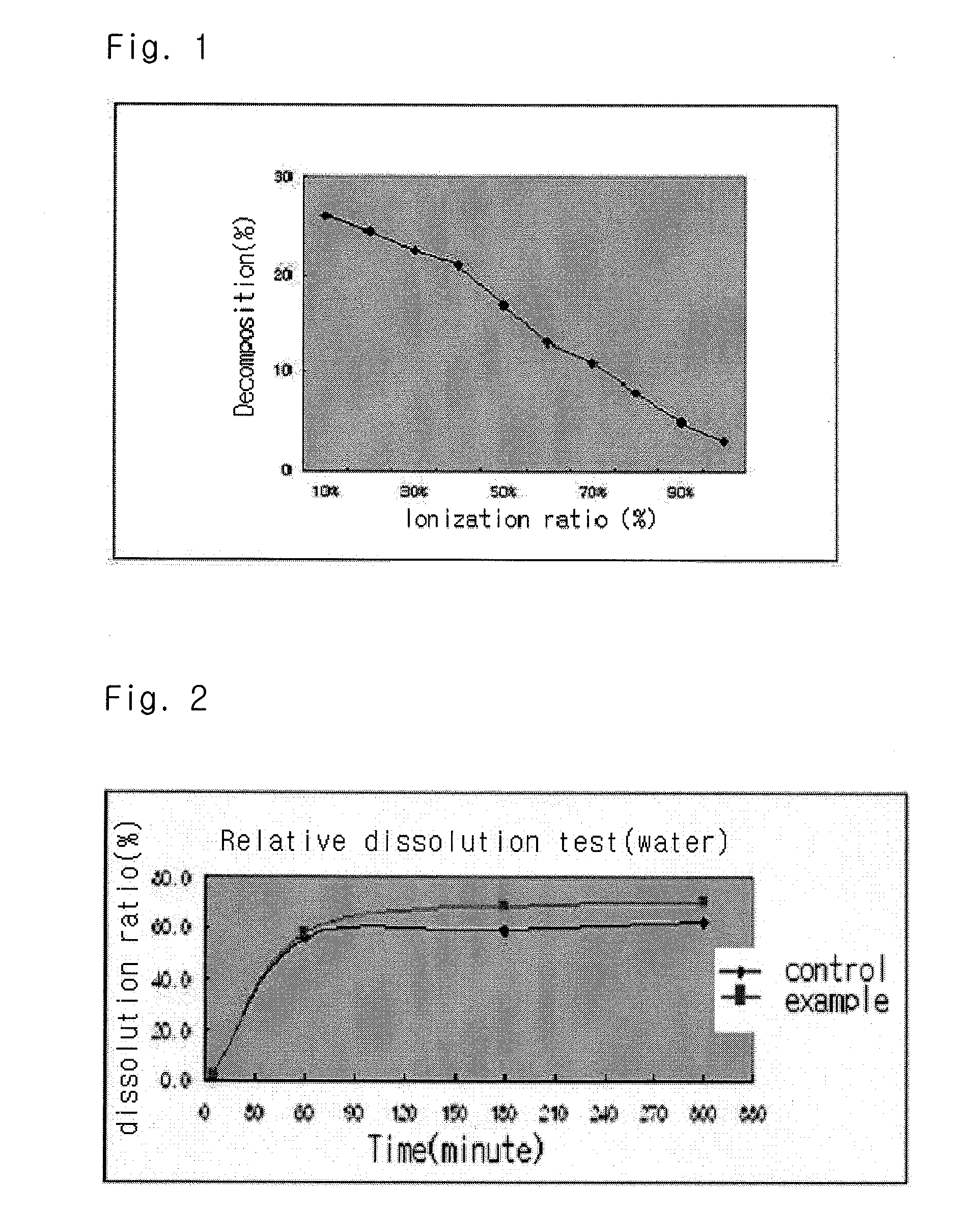
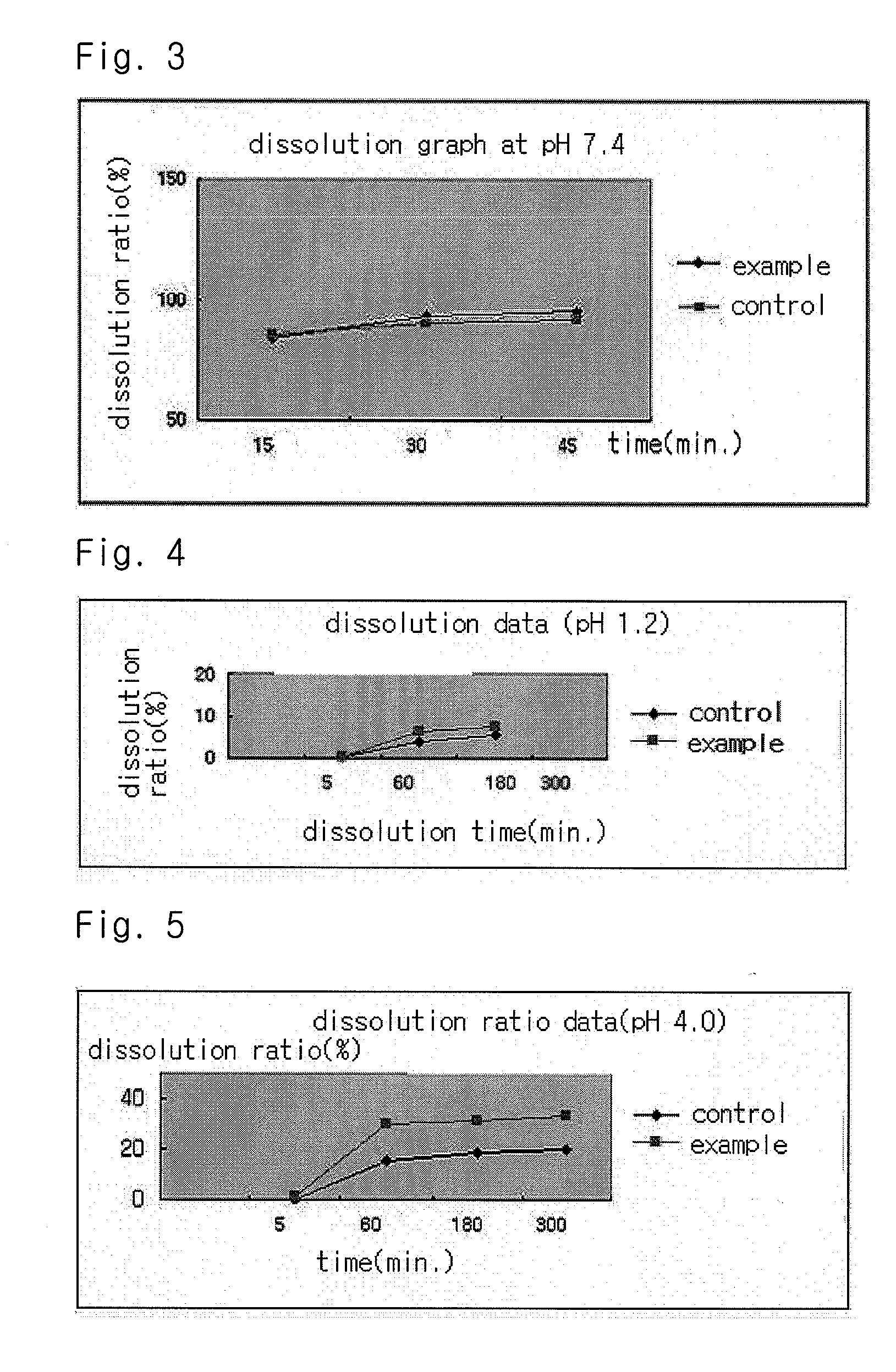
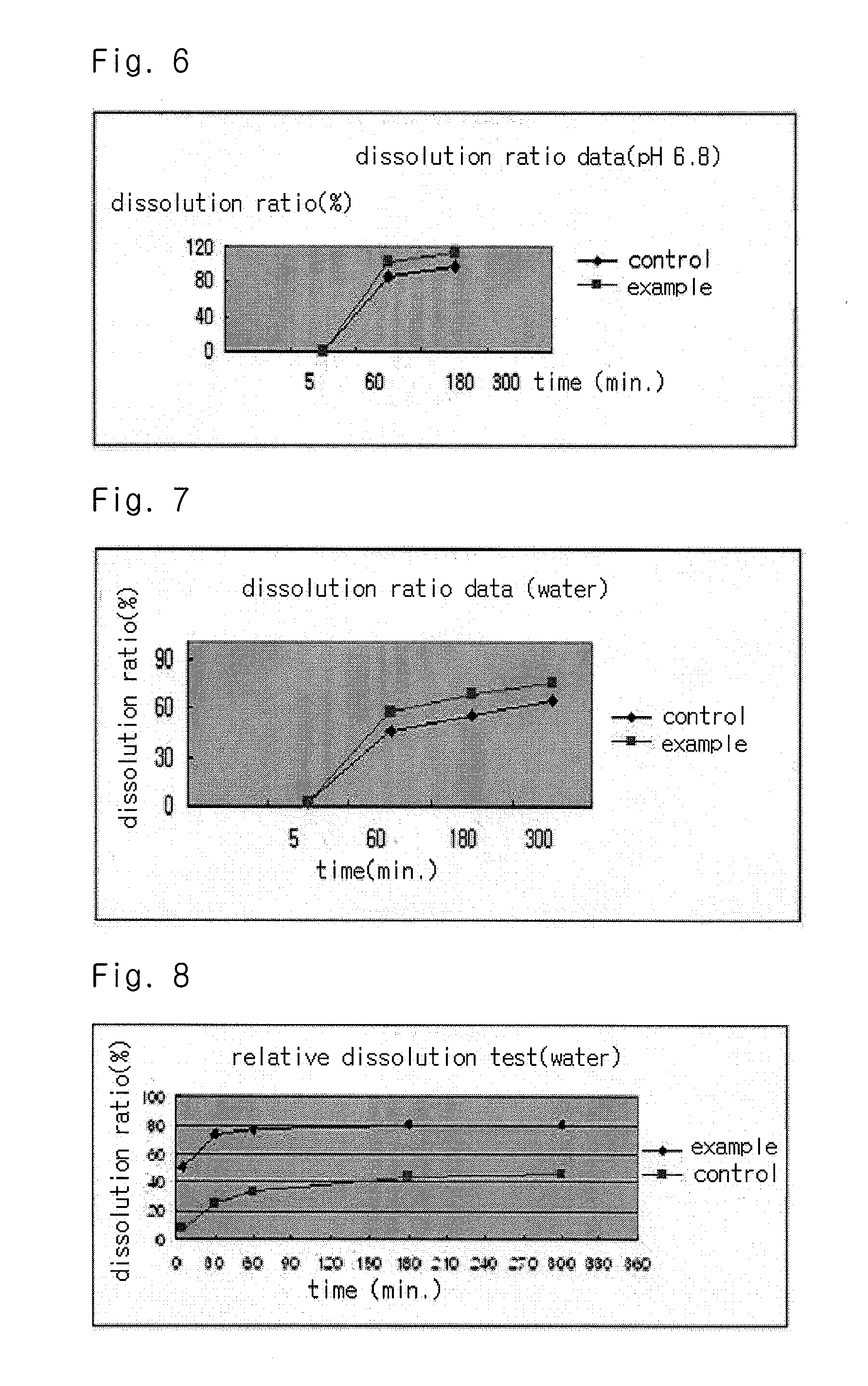
The present invention relates to a solvent system with improved disintegration degree and dissolution ratio of a hardly soluble drug by highly concentrating the drug through partial ionization, and by establishing optimal conditions for enhancing bioavailability of the drug, such as the co-relation between the acid drug and the accompanied components, ionization degree of a solvent system, use of an appropriate cation acceptor, water content, selection of optimal mixing ratio of the respective components and use of specific surfactants, and to a pharmaceutical preparation comprising the same. The solvent system of the invention has advantages in that it can enhance bioavailability by improving the disintegration degree and dissolution ratio of a hardly soluble drug and also provide a capsule with a sufficiently small volume to permit easy swallowing.
Owner:R & P KOREA
Non-addictive analgesic sustained-release drug delivery system and preparation method thereof
ActiveUS20150359891A1Improve drug deliveryExcellent mutual solubilizationBiocideNervous disorderEthyl lactateBENZYL ALCOHOL/WATER
A non-addictive analgesic sustained-release drug delivery system, comprising: (1) a narcotic analgesic drug having a concentration of 1 mg / ml-160 mg / ml, the drug being selected from a group consisting of: a local analgesic drug, and the combination of the local analgesic drug and a nonsteroidal analgesic drug and / or an opioid analgesic drug; (2) a drug menstruum in a proportion of 1%-75% (v / v), the menstruum being selected from a group consisting of benzyl alcohol, ethanol, benzyl benzoate, ethyl lactate, and tetrahydrofurfuryl polyethylene glycol ether; and (3) a drug sustained-release formulation having a proportion of 25%-99% (v / v), the sustained-release formulation being selected from a group consisting of natural vegetable oil, synthetic lipid, artificially improved half-natural lipid and derivative thereof. Also disclosed are a preparation process and use of the sustained-release drug delivery system.
Owner:LIPONT PHARMA
Nanometer particle of insoluble medicine and its prepn
InactiveCN1771910AThe composition of the prescription is simpleThe method is reliable and practicalAntibacterial agentsPowder deliveryZeta potentialDrugs solution
The present invention provides one kind of nanometer particle of insoluble medicine and its preparation process. The nanometer particle has size smaller than 1000 nm, negative charge and zeta potential of 0-30 mV, and consists of medicine in 0.1-100 wt% and protecting agent in 0-99.9 wt%. The medicine solution forms emulsified drop in water medium containing surfactant and / or emulsified drop protecting agent through í‹solvent diffusioníŒ mode, and is homogenized to form nanometer medicine particle via the common effect of mechanical force and medicine solvent in í‹high pressure emulsifying homogenizationíŒ mode. The preparation process is reliable and practical, has common medicine supplementary material, including lecithin, poloxamer 188, etc., and is suitable for production process of nanometer medicine particle.
Owner:ZHEJIANG UNIV
Transdermal drug administering system
InactiveCN1663560ALow costReduce wasteMedical devicesPharmaceutical non-active ingredientsVerapamil HydrochlorideWater soluble drug
The invention discloses a percutaneous give drug system, which comprises: a drug storeroom, the gel drug layer comprises drug, solvent, transdermal accelerating agent and gelatinizing agent; one adsorption layer comprised solvent absorption material of solvent in absorbable drug storeroom; one isolating layer between drug storeroom and adsorption layer, which is of non-woven fabrics or semipermeable membrane to make solvent of gas or liquid pass through successfully; adsorption layer can joint isolating layer of drug storeroom in opposite directions of closing up to skin of drug storeroom. The percutaneous give drug system in this invention can increase drug level in drug storeroom of the system to elevate drug transdermal speed, as well as increase controlled release effect of percutaneous give drug and absolute bioavailability, enhance drug curative effect, decrease drug waste, and depress preparation cost of the system. The invention is fit particular to percutaneous give drug system for water-soluble drug of propranolol hydrochloride, verapamil hydrochloride and chlorpromazine hydrochloride.
Owner:HANGZHOU MINSHENG PHARM CO LTD
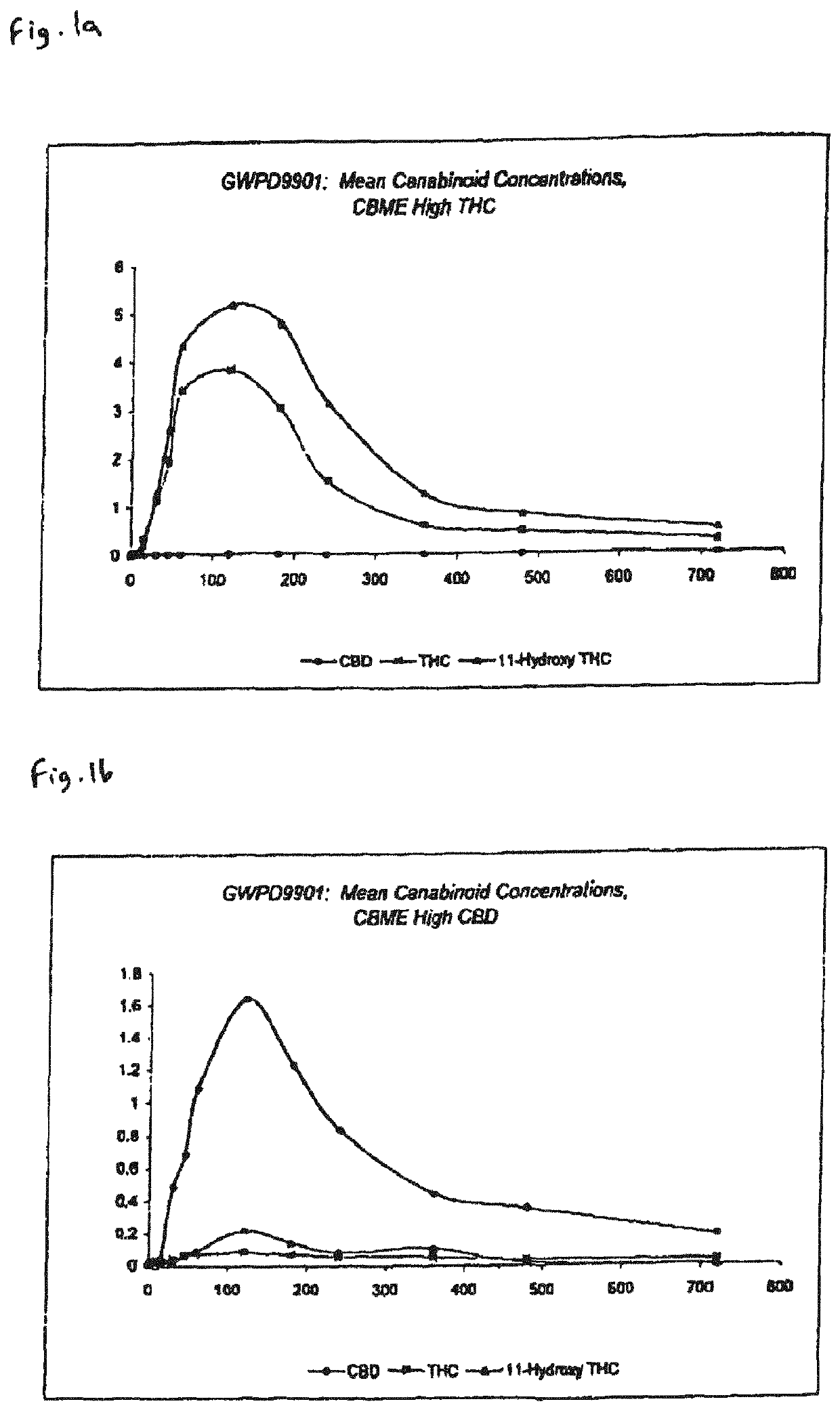
Pharmaceutical formulation
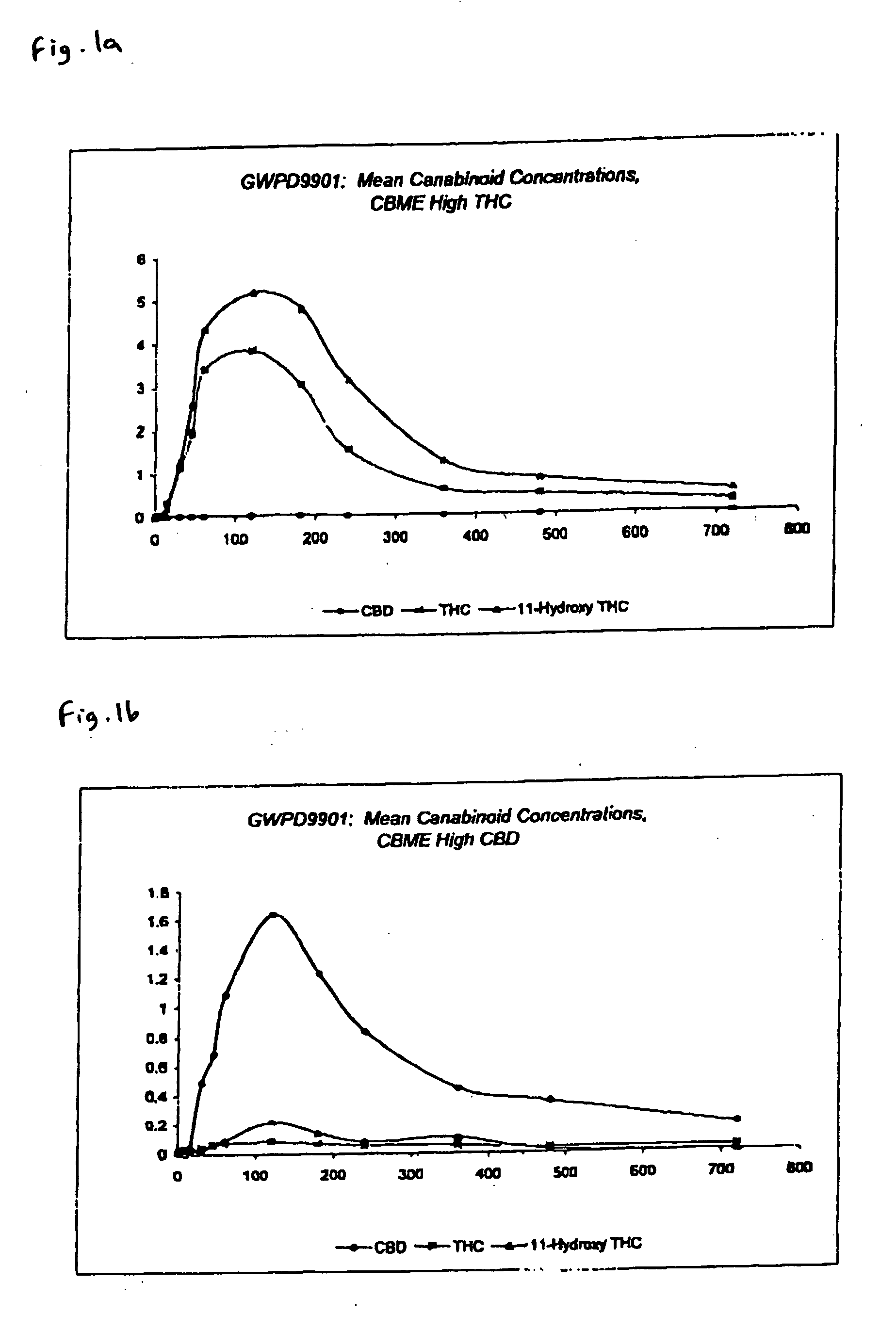
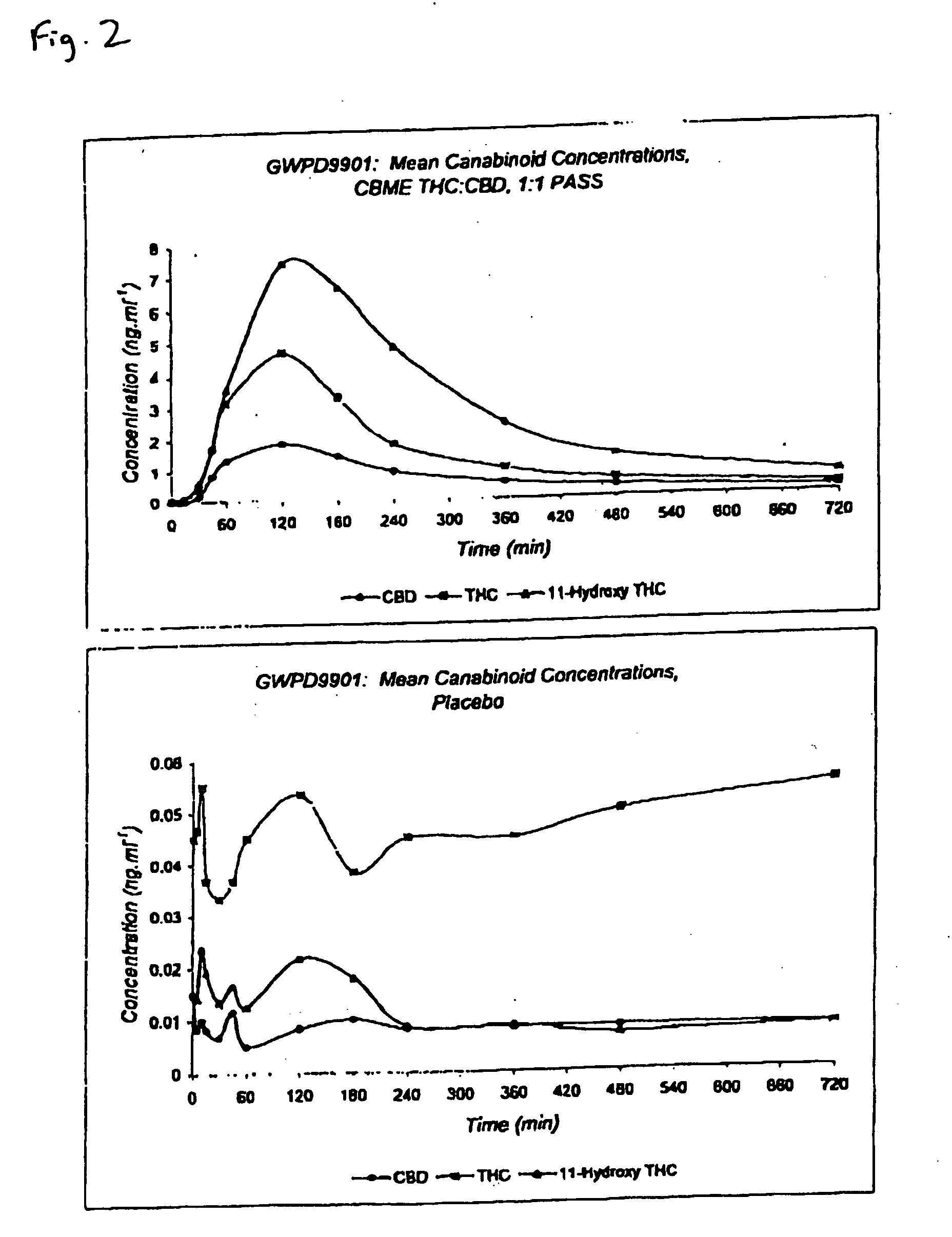
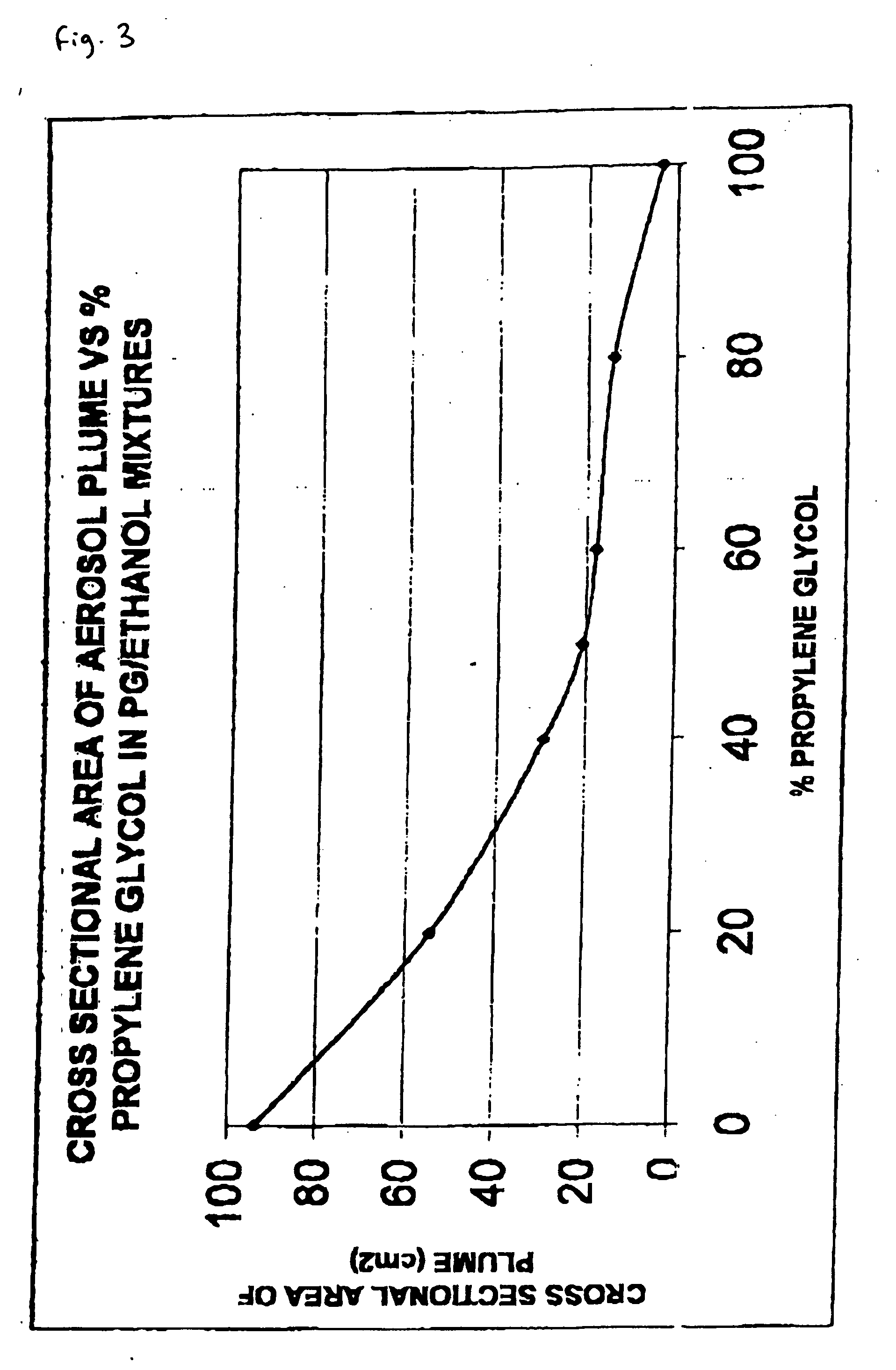
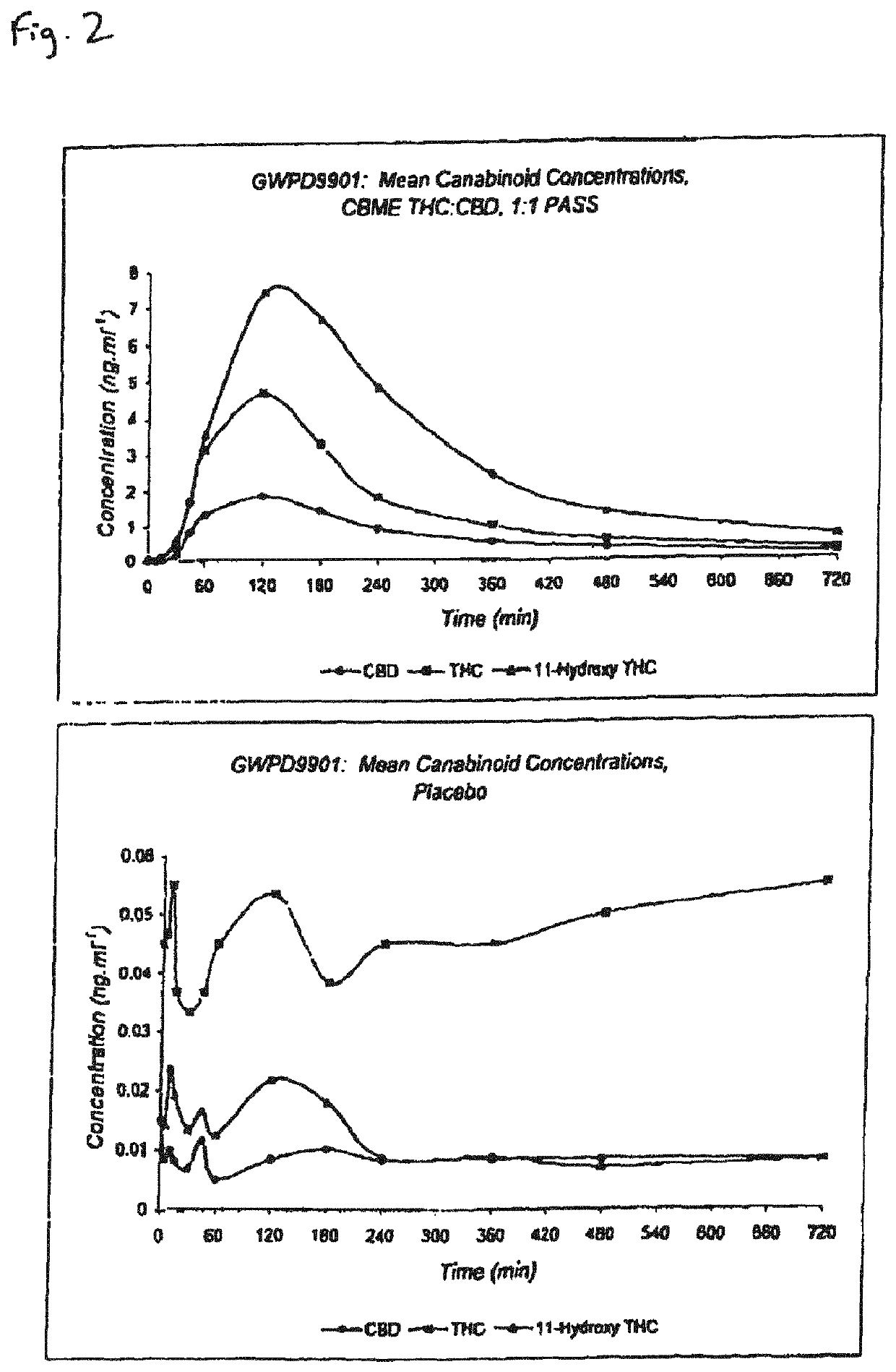
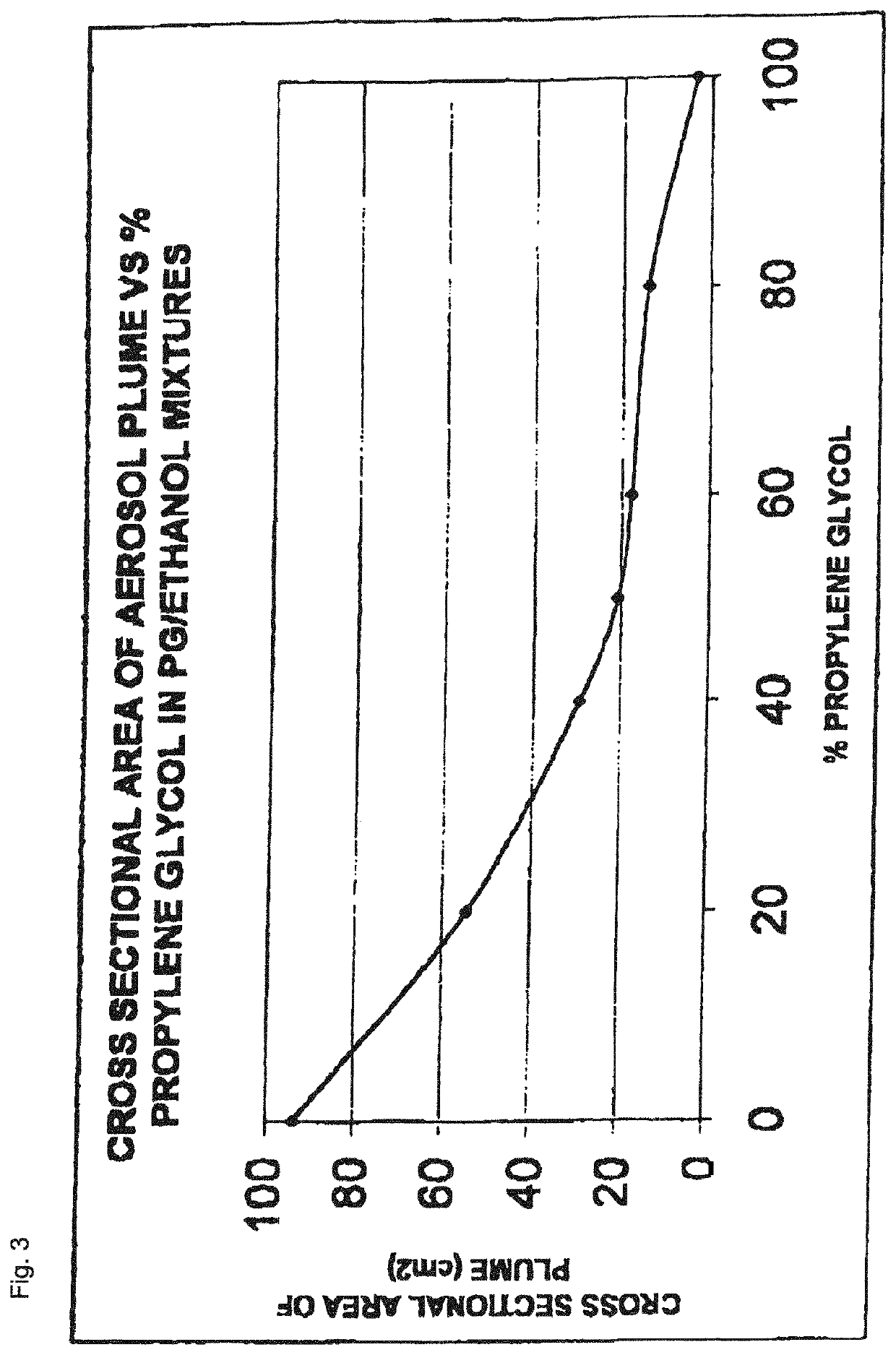
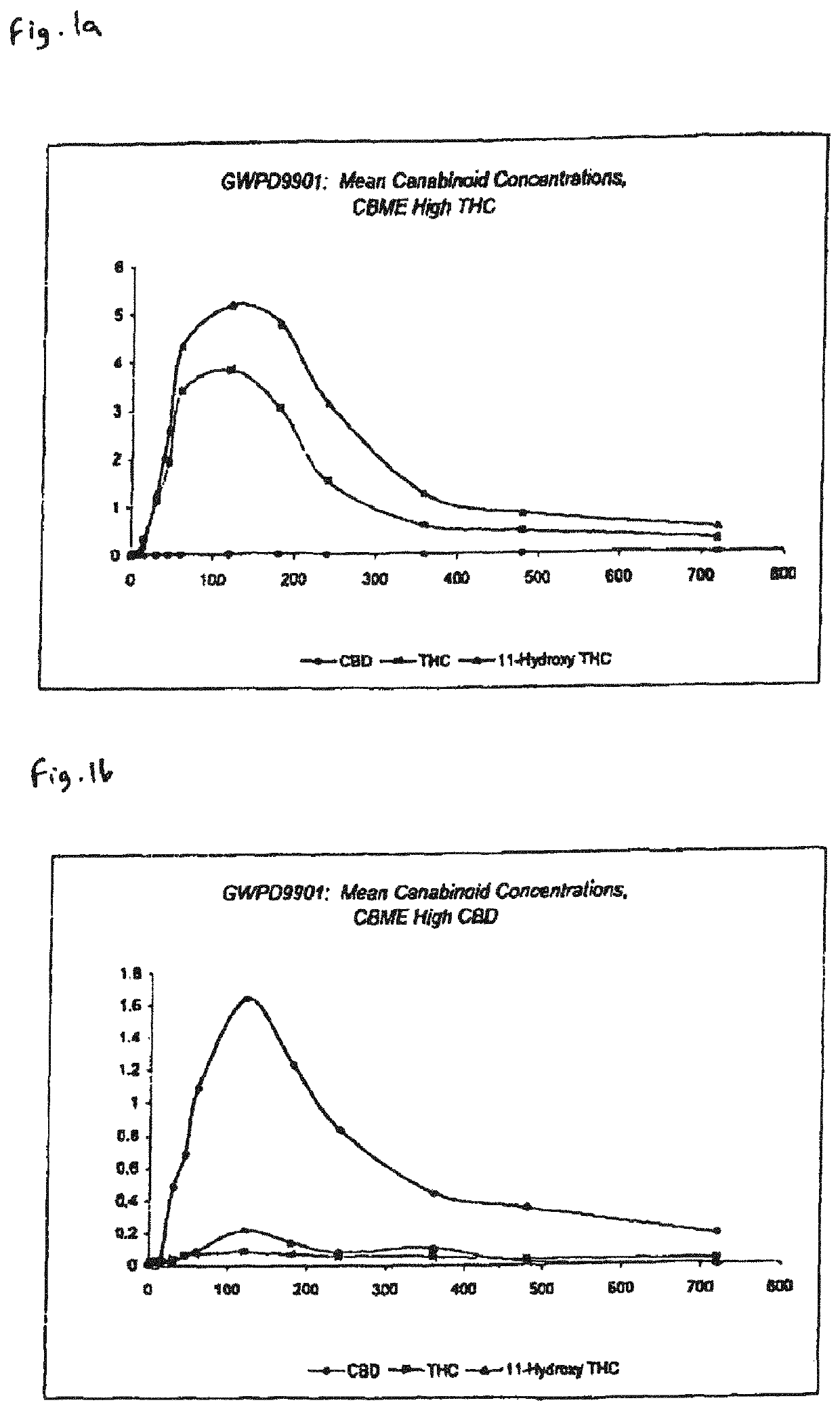
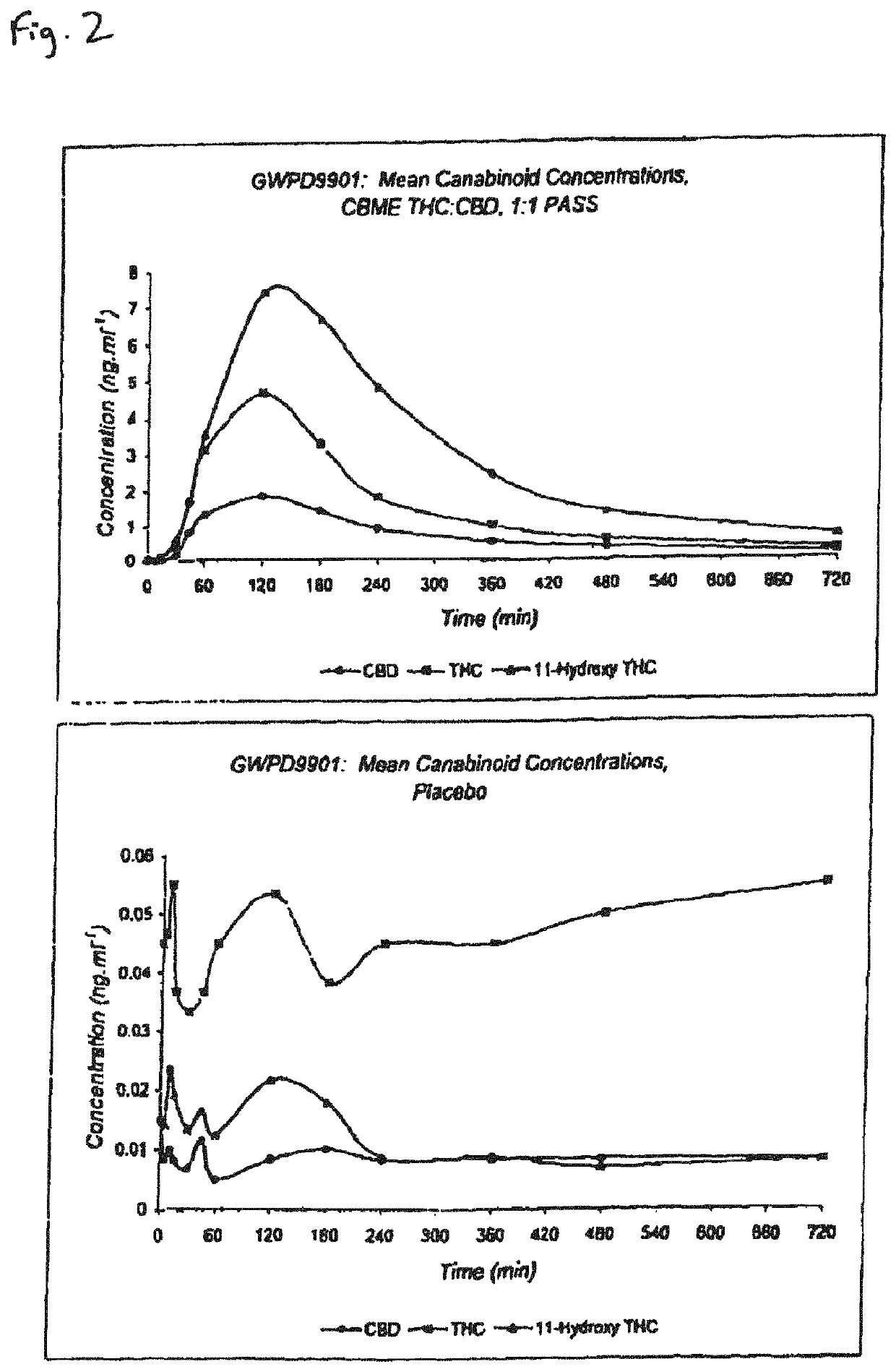
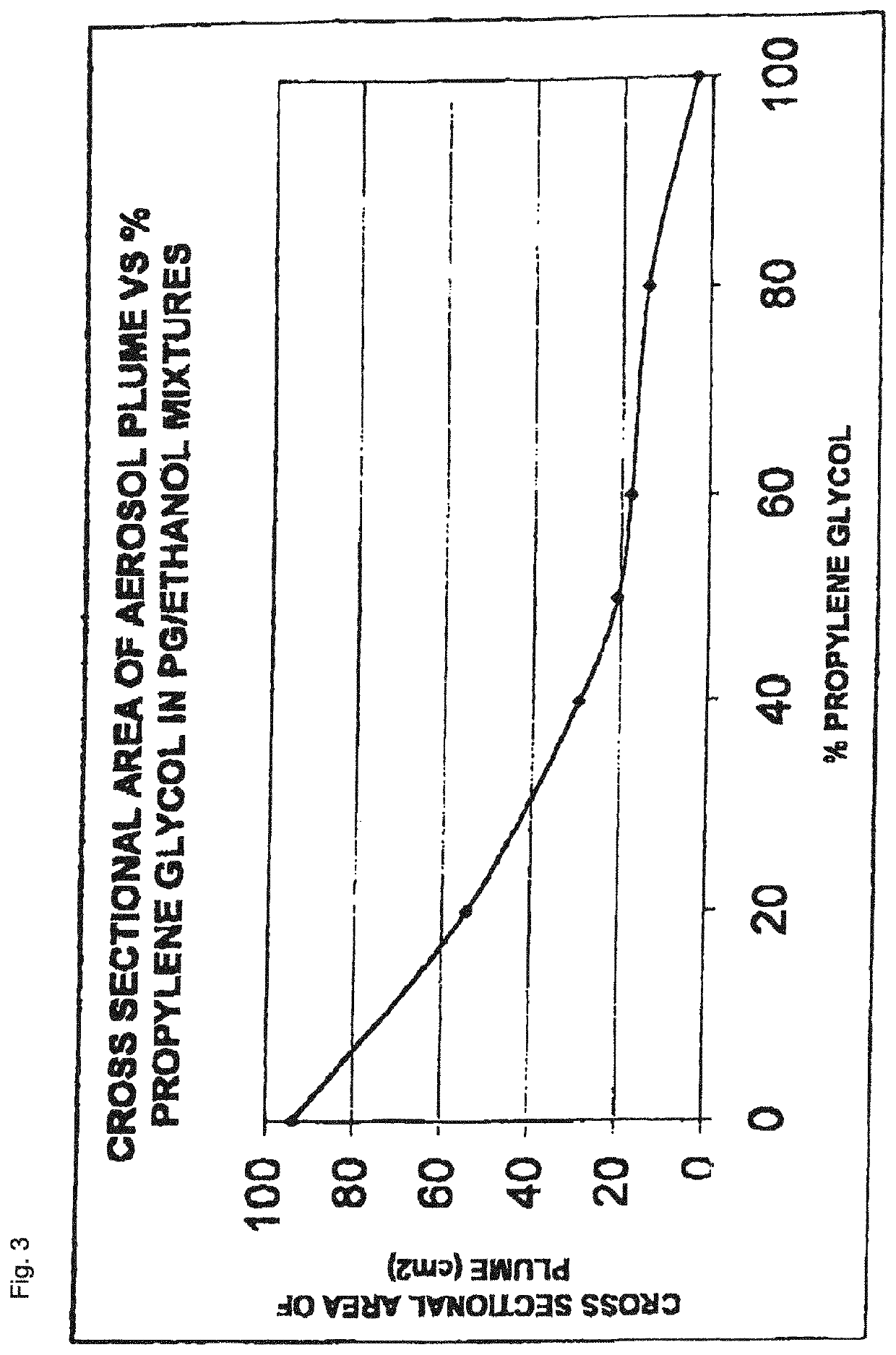
The invention relates to pharmaceutical formulations, and more particularly to formulations containing cannabinoids for administration via a pump action spray. In particular, the invention relates to pharmaceutical formulations, for use in administration of lipophilic medicaments via mucosal surfaces, comprising: at least one lipophilic medicament, a solvent and a co-solvent, wherein the total amount of solvent and co-solvent present in the formulation is greater than 55% wt / wt of the formulation and the formulation is absent of a self emulsifying agent and / or a fluorinated propellant.
Owner:GW RES LTD
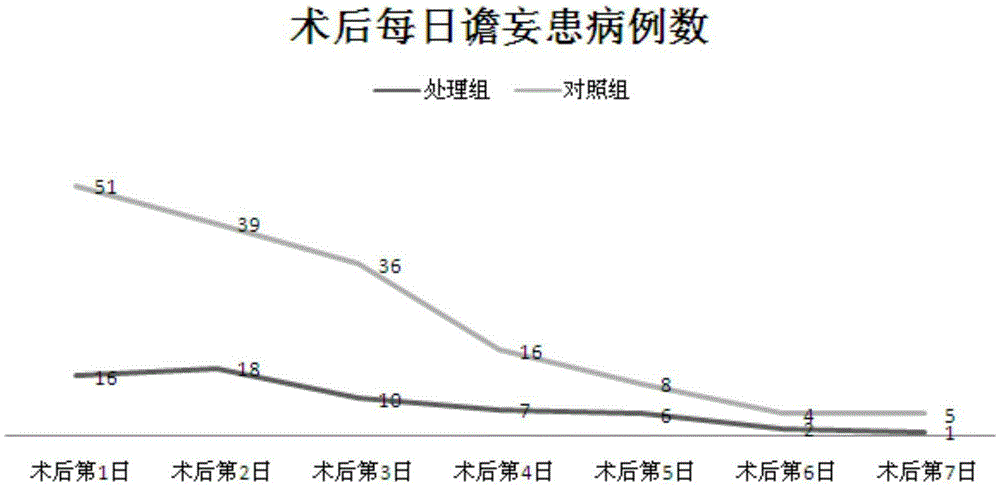
Pharmaceutical composition preventing postoperative delirium
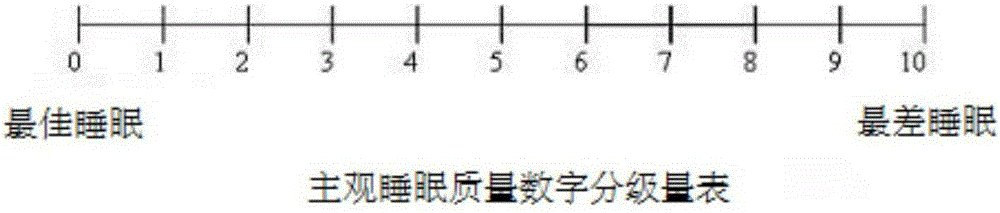
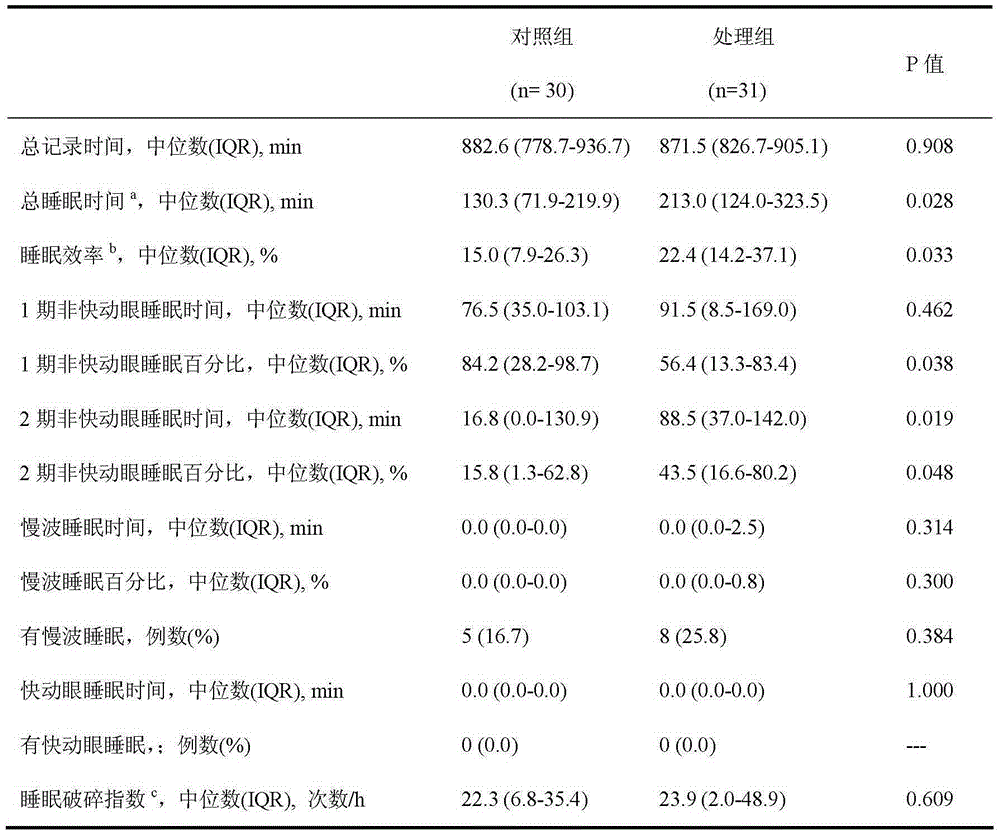
InactiveCN105287519ATotal sleep time increasedImprove subjective sleep qualityOrganic active ingredientsNervous disorderDexmedetomidineSedative
The invention relates to the field of pharmaceutical compositions, and especially relates to a pharmaceutical composition preventing postoperative delirium. The provided pharmaceutical composition contains a pharmaceutical solvent carrier and dexmedetomidine or pharmaceutically-acceptable salts dissolving in the solvent carrier. The pharmaceutical composition is applicable to improve postoperative patient sleep structure, thereby improving patient sleep quality. The pharmaceutical composition is capable of preventing postoperative patient delirium, also is capable of mitigating patient pain, is safe to use and low in cost, and is usable by being matched with one or more of other sedatives, narcotics, hypnotics and opioids.
Owner:PEKING UNIV FIRST HOSPITAL
Lacosamide oral solution and preparation method thereof
InactiveCN106551900AGreat tasteFast absorptionOrganic active ingredientsNervous disorderMedicinePreservative
The invention discloses a lacosamide oral solution and a preparation method of the lacosamide oral solution and belongs to the field of pharmaceutical preparations. The lacosamide oral solution comprises lacosamide, an appropriate medicine solvent system, one or more flavoring agents, one or more preservatives, one or more acid-base modifiers, one or more solubilizers and one or more thickeners. The lacosamide oral solution is stable in performance, good in taste, rapid in absorption, capable of acting rapidly and good in curative effect.
Owner:BEIJING VENTUREPHARM BIOTECH
Adhesive peel-forming formulations for dermal delivery of drugs and methods of using the same
ActiveUS8907153B2Easily peelableEasily removablePowder deliveryOrganic active ingredientsSolubilitySkin surface
The present invention is drawn to adhesive peel-forming formulations for dermal delivery of a drug. The formulation can include a drug, a solvent vehicle, and a peel-forming agent. The solvent vehicle can include a volatile solvent system having one or more volatile solvent, and a non-volatile solvent system having one or more non-volatile solvent, wherein the non-volatile solvent system has a solubility for the drug that is within a window of operable solubility for the drug such that the drug can be delivered at therapeutically effective rates over a sustained period of time. The formulation can have a viscosity suitable for application to a skin surface prior to evaporation of the volatile solvents system. When applied to the skin, the formulation can form a solidified peelable layer after at least a portion of the volatile solvent system is evaporated.
Owner:CRESCITA THERAPEUTICS INC
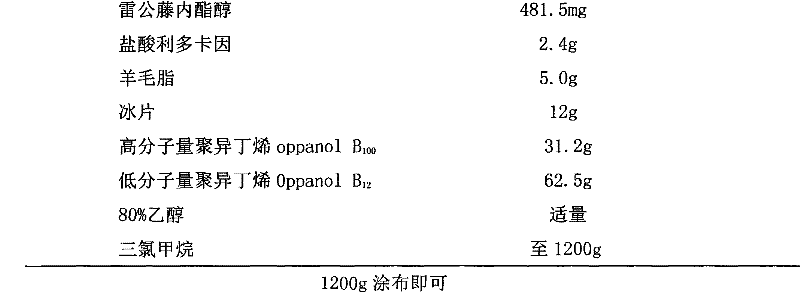
Patch for treating rheumatic arthritis and preparation method thereof
InactiveCN102670624AEasy to useNo oralOrganic active ingredientsAntipyreticControlled releaseDisease
The invention discloses a patch for treating rheumatic arthritis and a preparation method thereof. The patch consists of a main medicament for treating rheumatic arthritis, a medicament solvent, a substrate and a penetrating agent, wherein the main medicament consists of triptolide and lidocaine serving as a pain-relieving medicament for relieving rheumatic arthritis pain. The patch is medical controlled-release slow release preparation, has a comprehensive pharmacological action, is used for blocking major development pathologic loops of the rheumatic arthritis disease, and has high specificity and a lasting pharmacological effect; and the preparation method is simple.
Owner:FUJIAN ACAD OF MEDICAL SCI
Pharmaceutical formulation
InactiveUS10538373B2The process is simple and effectiveMaximize contactNervous disorderFlexible coversPharmaceutical formulationPharmaceutical Substances
The invention relates to pharmaceutical formulations, and more particularly to formulations containing cannabinoids for administration via a pump action spray. In particular, the invention relates to pharmaceutical formulations, for use in administration of lipophilic medicaments via mucosal surfaces, comprising: at least one lipophilic medicament, a solvent and a co-solvent, wherein the total amount of solvent and co-solvent present in the formulation is greater than 55% wt / wt of the formulation and the formulation is absent of a self emulsifying agent and / or a fluorinated propellant.
Owner:GW RES LTD
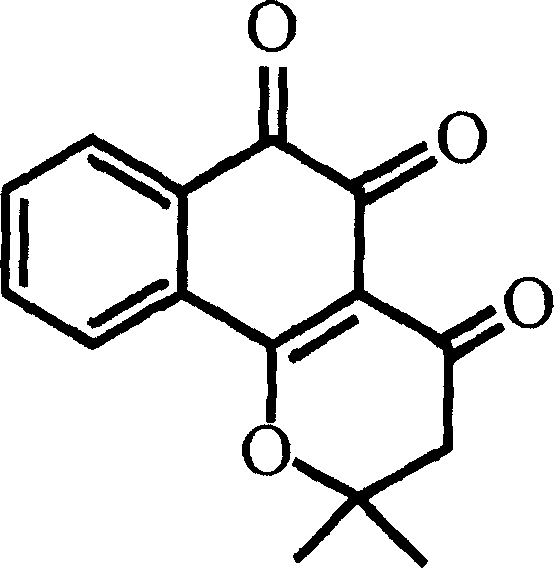
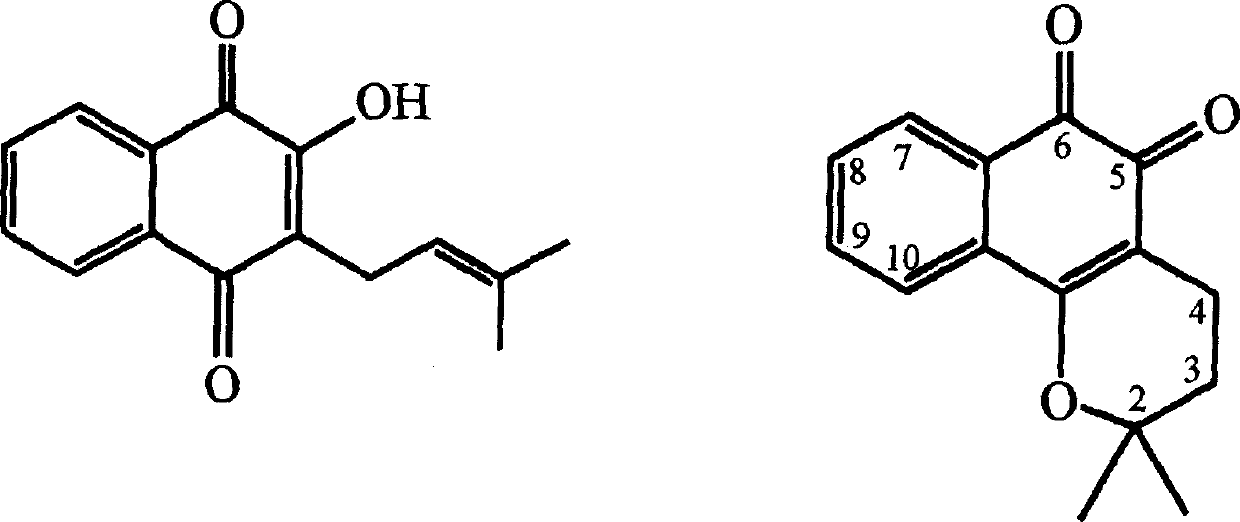
Pharmaceutical compositions containing beta-lapachone, or derivatives or analogs thereof, and methods of using same
It has been confirmed that β-paradone has potent antitumor activity and inhibits human cancer lines, but its solubility in most pharmaceutical solvents is poor. The present invention overcomes this important defect by adopting a novel pharmaceutical composition, which contains an effective amount of β-paradone or its derivatives, analogs and pharmaceutical solubilizing carrier molecules, and the solubilizing carrier molecules can be It is a water-solubilizing carrier molecule, such as hydroxypropyl-β-cyclodextrin, or an oil-based solubilizing carrier molecule to enhance the solubility of β-paradone in an aqueous solution. An effective amount of β-paradone or its derivatives and analogs can be mixed with pharmaceutical solubilizing carrier molecules in an aqueous solution. The novel pharmaceutical compositions can be administered with a second anticancer agent, or in combination with radiation therapy. The invention also discloses a formula, which mixes pharmaceutical solubilizing carrier molecules with β-paladone or its derivatives and analogues, and after the mixture is freeze-dried, it is reconstituted in an aqueous solution to have effective solubility. The invention also provides the β-paladone emulsion in the excipient of the medicinal fat emulsion. The invention also discloses a method for treating cancer by administering the novel pharmaceutical composition and formulation to a sick body. The invention also provides a pharmaceutical kit.
Owner:阿奎利公司
Qingkailing prepn for great transfusion and its prepn process
InactiveCN101019949AOvercoming low clarityOvercome stabilityOrganic active ingredientsAntipyreticHyodeoxycholic acidHydrolysis
The Qingkailing preparation for great transfusion is prepared with cholic acid 1.2-2.0 weight portions, hyodeoxycholic acid 1.4-2.4 weight portions, buffalo horn 9-16 weight portions, baicalin 1.8-3.2 weight portions, nacre 18-32 weight portions, cape jasmine 9-16 weight portions, isatis root 75-125 weight portions, and honeysuckle 23-38 weight portions. It is prepared through extraction, hydrolysis, mixing, filtering, active carbon treatment, sterilizing and other steps. The Qingkailing preparation for great transfusion has high clarity, high stability, high safety and other advantages, and is suitable for medical care personnel to use.
Owner:TSINGHUA UNIV
Contraceptive and preparation method thereof
InactiveCN105796572AIncreased sensitivityIncrease dependenceAerosol deliveryAntisepticsHormone drugPharmaceutical formulation
The invention relates to a contraceptive and a preparation method thereof. The present invention provides a hormonal contraceptive, and a chemical contraception prescription. An oral contraceptive hormone is dissolved or dispersed in an organic pharmaceutical solvent to prepared a stock solution; an external contraceptive is dissolved in purified water or a pharmaceutically acceptable solid carrier to dissolve the drug into a preparation; and the stock solution containing the oral contraceptive and prepared mixture containing external contraceptive are mixed. The present invention overcomes the respective defects of oral contraceptive and external contraceptive. The contraceptive takes the respective advantages of the oral and external contraceptives, and uses good drug absorption of the female vagina, especially sensitivity and dependence on hormone drugs to combine the characteristics of oral contraceptive and external contraceptive. The contraceptive creatively combines the two to avoid drug stimulation on the gastrointestinal tract, avoid the drug inactivation caused by liver first-pass metabolism, also reduce the dose, and form a novel pharmaceutical prescription.
Owner:郭曙平
Device for storing, extemporaneously preparing, and administering
InactiveCN102006850AInstant Mixing GuaranteedClosure with auxillary devicesPharmaceutical containersBiomedical engineeringLow volume
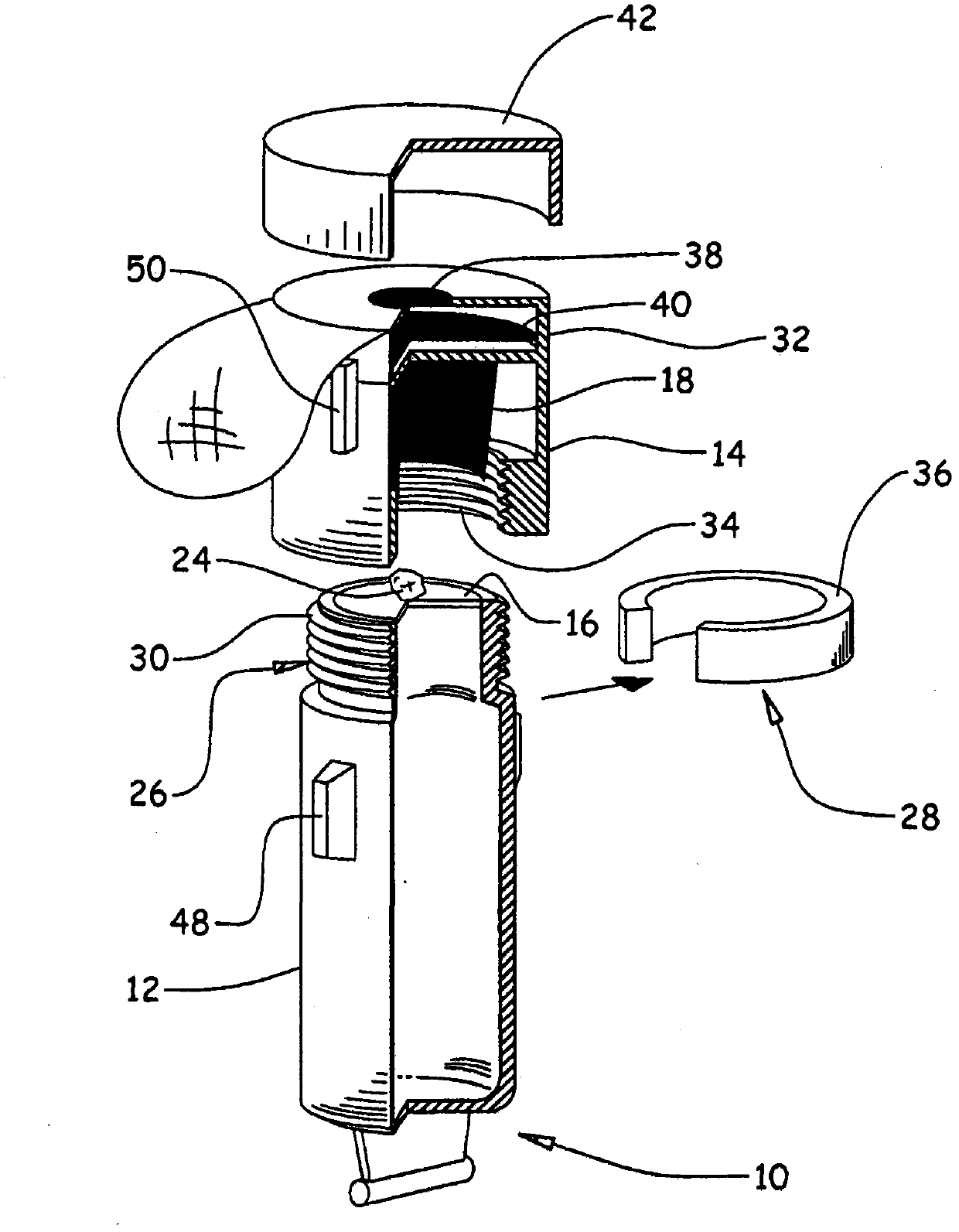
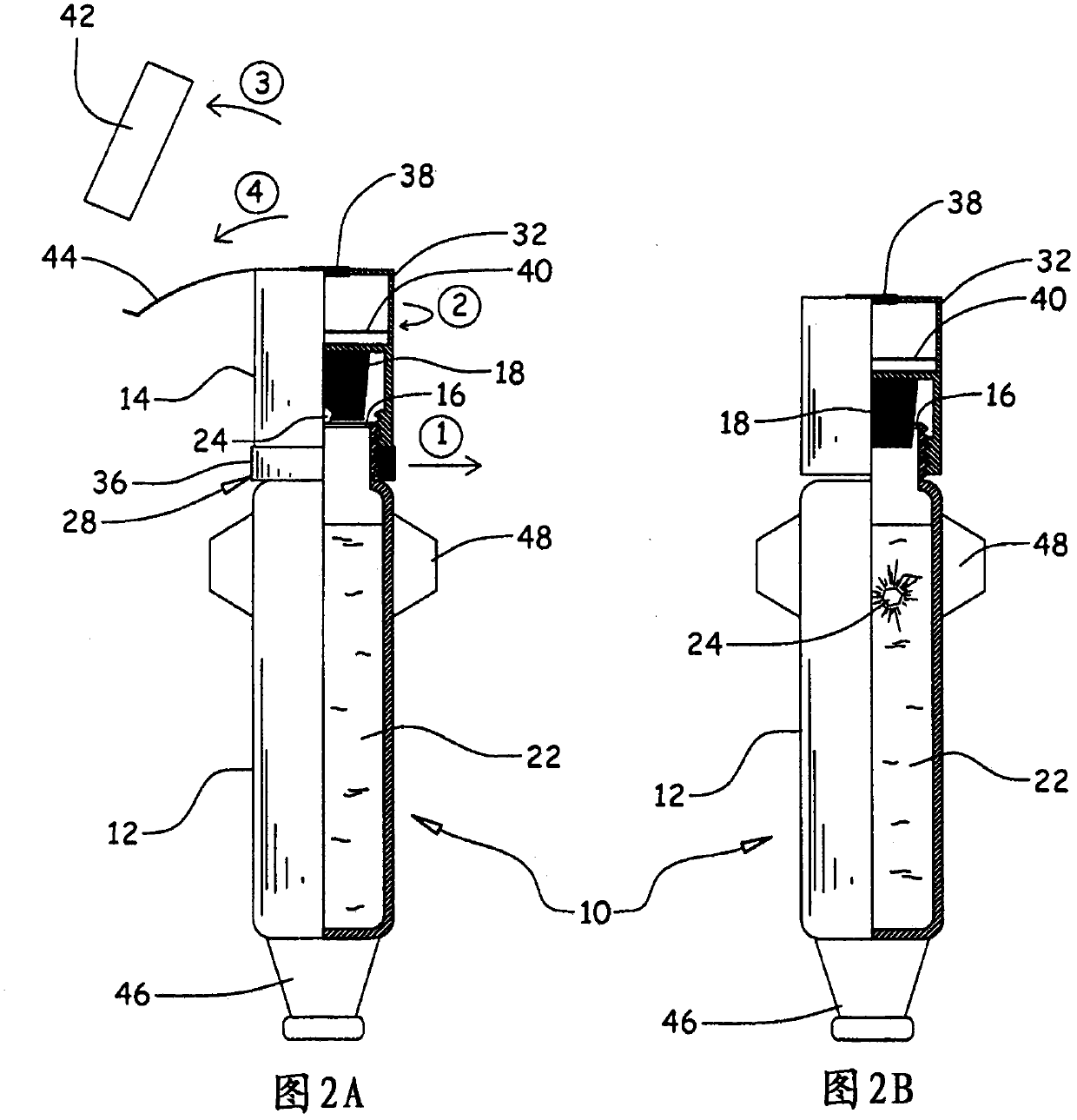
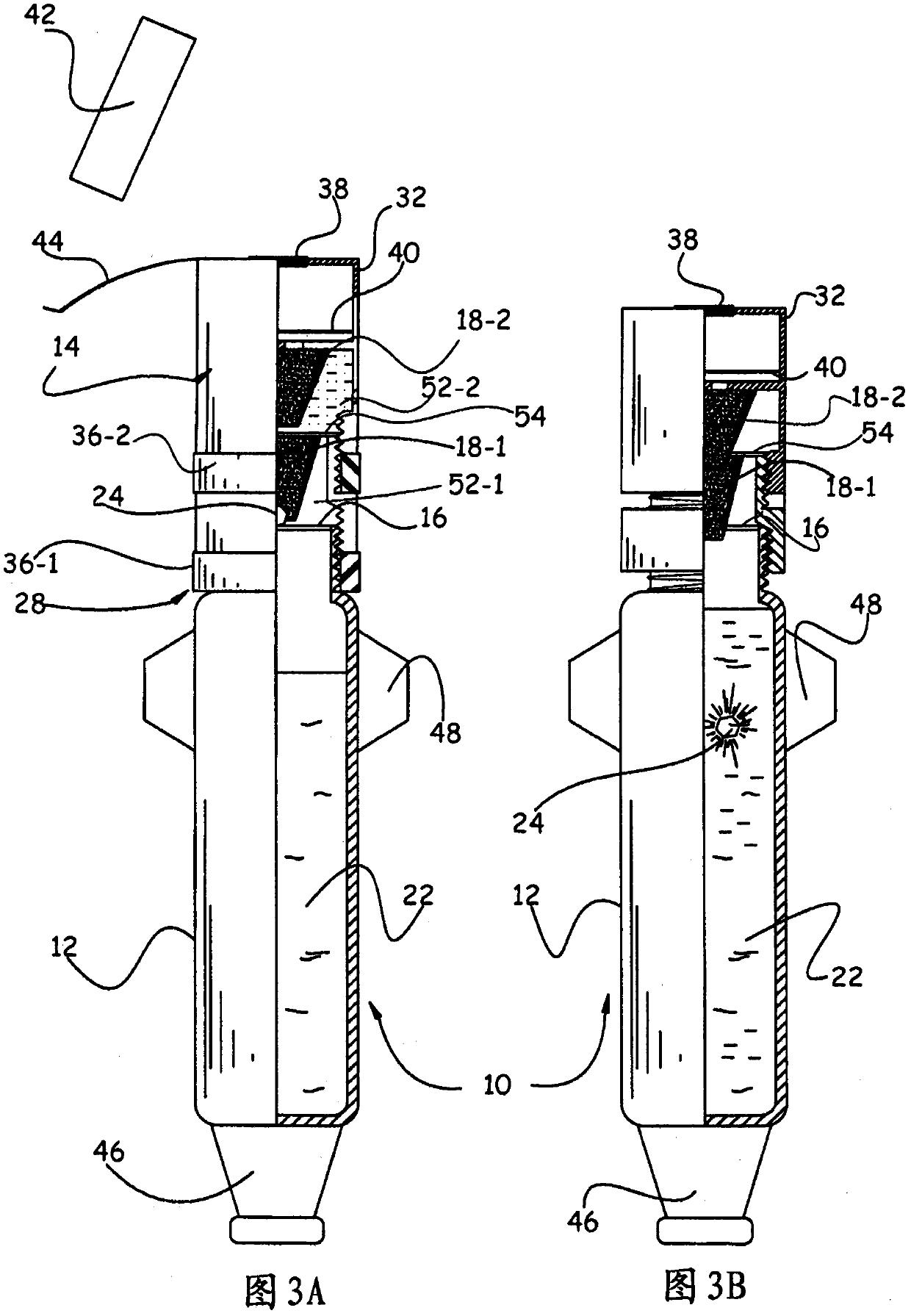
The invention relates to a device (10) for storing and extemporaneously preparing, before administration, at least one active principle, which includes: A body (12), comprising at least one compartment for containing at least one lower volume of 5 ml of pharmaceutical solvent (22); a head (14), comprising at least one compartment for containing at least one active principle (24), particularly a very low dose, and comprising, in the upper portion thereof, of at least one collection chamber (32) provided with a filter (40), said head (14) capable of taking a first storing position P1, wherein said head (14) is in a distal position relative to the body (12), and a second preparation position P2, wherein said head (14) is in a proximal position relative to the body (12), at least one partition (16) separating the body (12) and the head (14); and a rupturing means (18) for rupturing said partition (16) such that the active principle (24) comes into contact with the solvent (22) and dissolves into the latter. The invention also aims to use said device to store and extemporaneously prepare at least one active principle with a view to administer the same.
Owner:菲利普·佩罗维奇
Pharmaceutical formulation
InactiveUS20200102131A1The process is simple and effectiveMaximize contactFlexible coversNervous disorderPharmaceutical formulationPharmaceutical Substances
The invention relates to pharmaceutical formulations, and more particularly to formulations containing cannabinoids for administration via a pump action spray. In particular, the invention relates to pharmaceutical formulations, for use in administration of lipophilic medicaments via mucosal surfaces, comprising: at least one lipophilic medicament, a solvent and a co-solvent, wherein the total amount of solvent and co-solvent present in the formulation is greater than 55% wt / wt of the formulation and the formulation is absent of a self emulsifying agent and / or a fluorinated propellant.
Owner:GW RES LTD
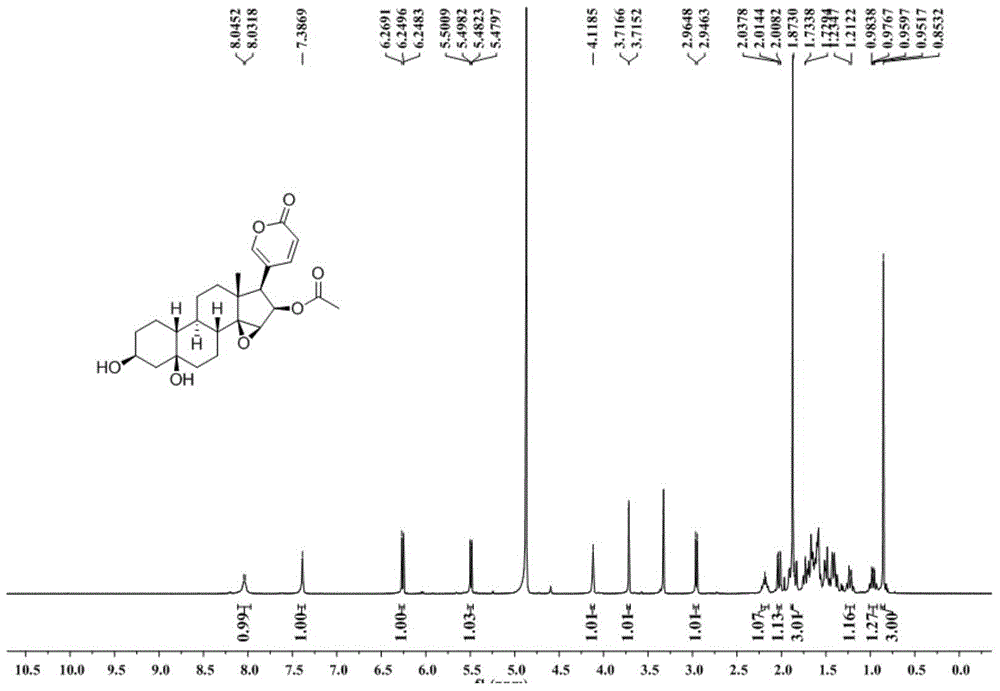
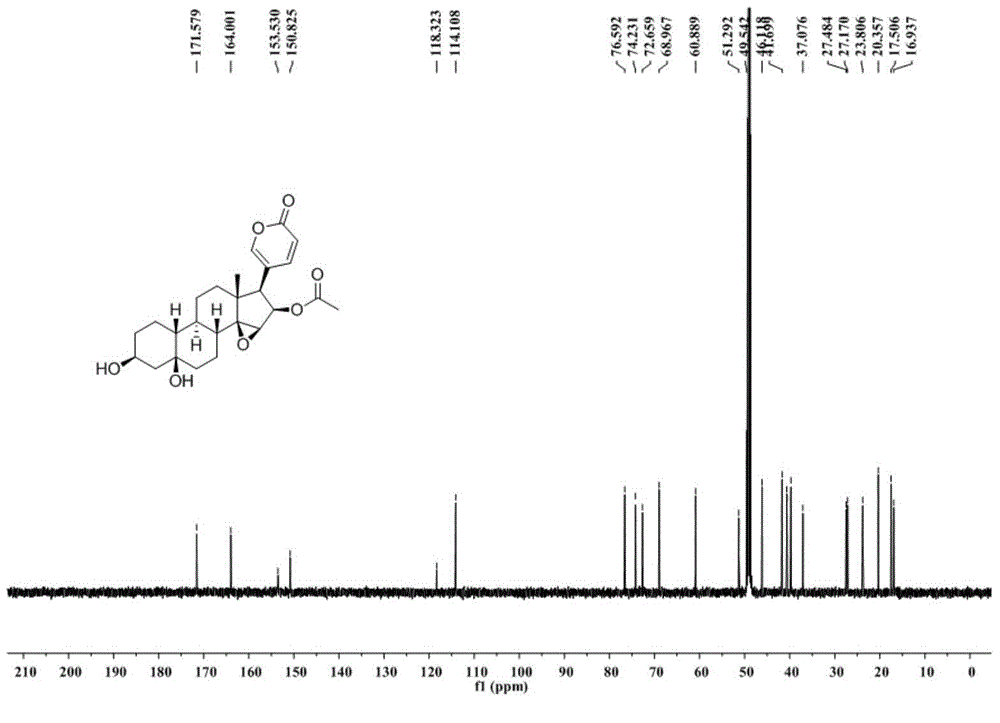
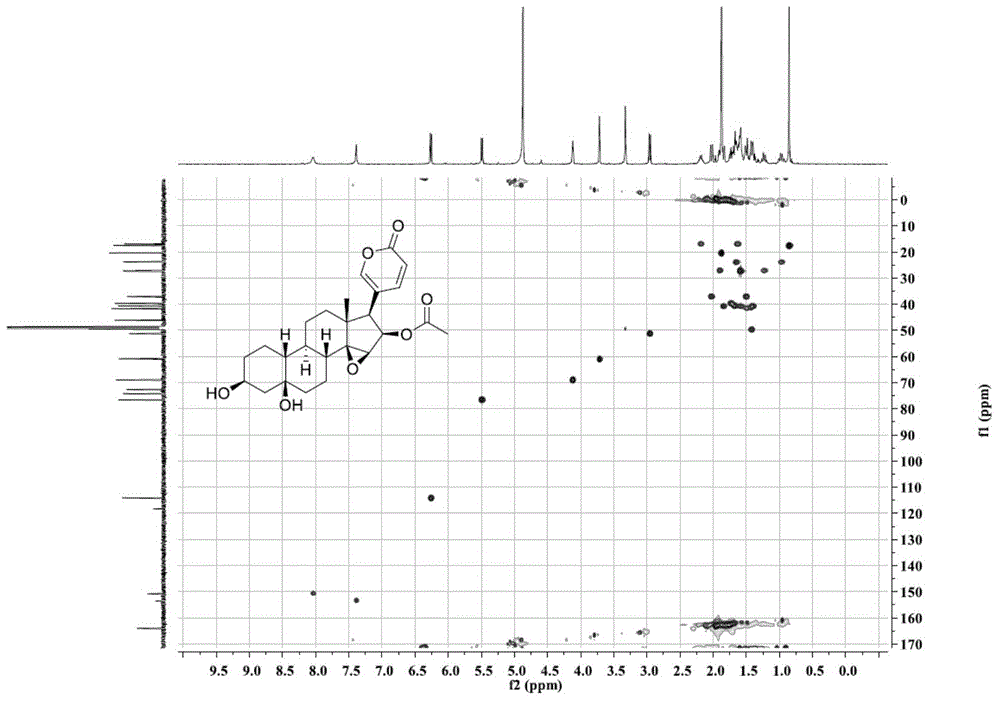
Novel 19-demethyl resibufogenin compound and application thereof to preparation of antitumor pharmaceutical preparation
ActiveCN104892721APrevent proliferationGood antitumor activityOrganic active ingredientsSteroidsSide effectPreservative
The invention relates to the field of natural drugs and chemical drugs and particularly relates to application of a novel 19-demethyl resibufogenin compound to preparation of an antitumor pharmaceutical preparation. The novel 19-demethyl resibufogenin compound has a structure as shown in the formula I, is named as Bufogargarin A and is extracted and separated from toad skin. The Bufogargarin A disclosed by the invention has remarkable antitumor activity in vitro and vivo and is not observed to have remarkable toxic or side effects under a therapeutic dose so as to have a favorable pharmaceutical prospect and be applied to preparation of the antitumor pharmaceutical preparation. The antitumor pharmaceutical preparation contains Bufogargarin A and the balance of pharmaceutical auxiliary materials or other compatible drugs; the pharmaceutical auxiliary materials can be various pharmaceutical solvents, disintegrating agents, flavoring agents, preservatives, colorants and binding agents; and the antitumor pharmaceutical preparation includes various clinical dosage forms.
Owner:JINAN UNIVERSITY
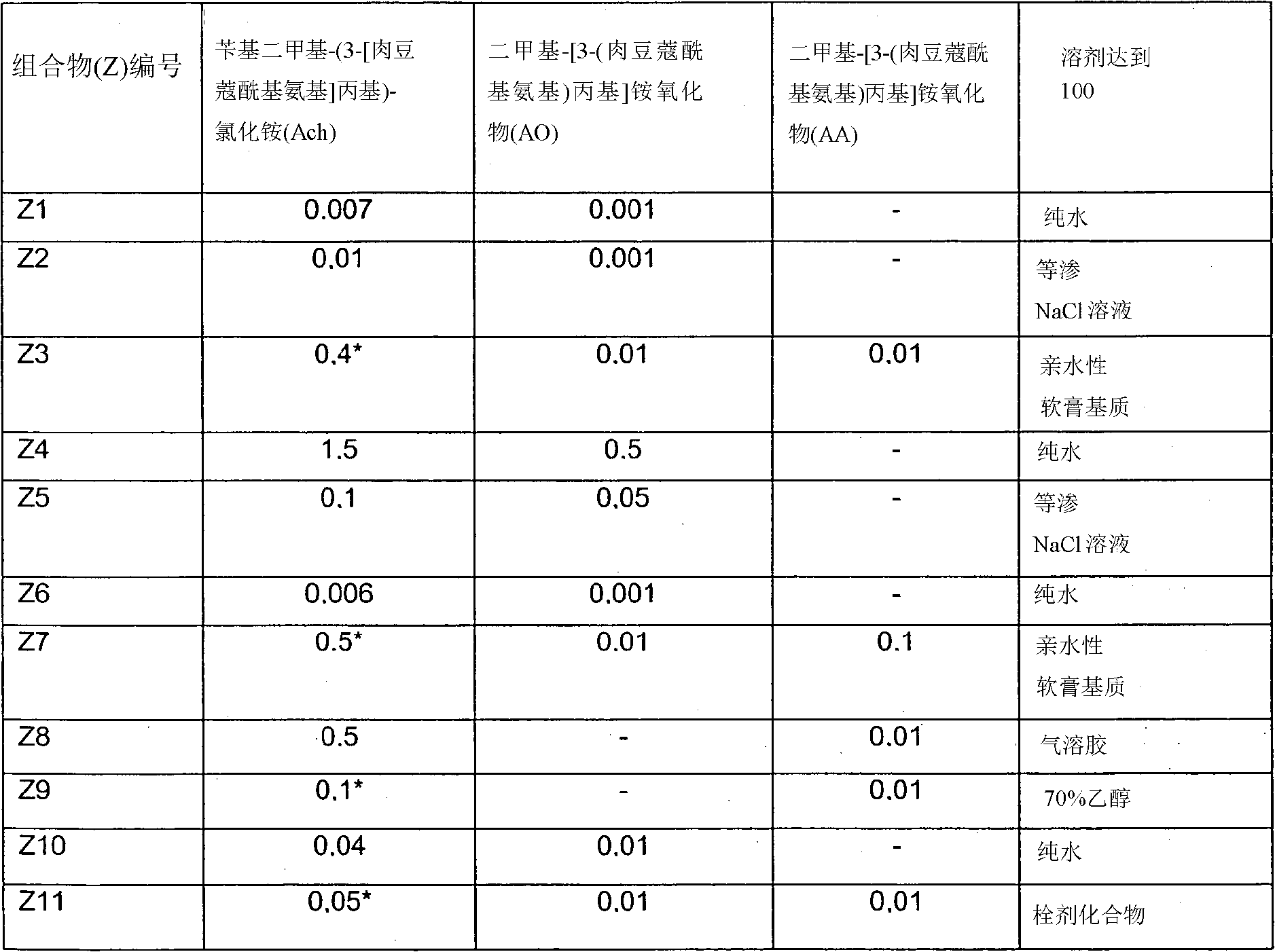
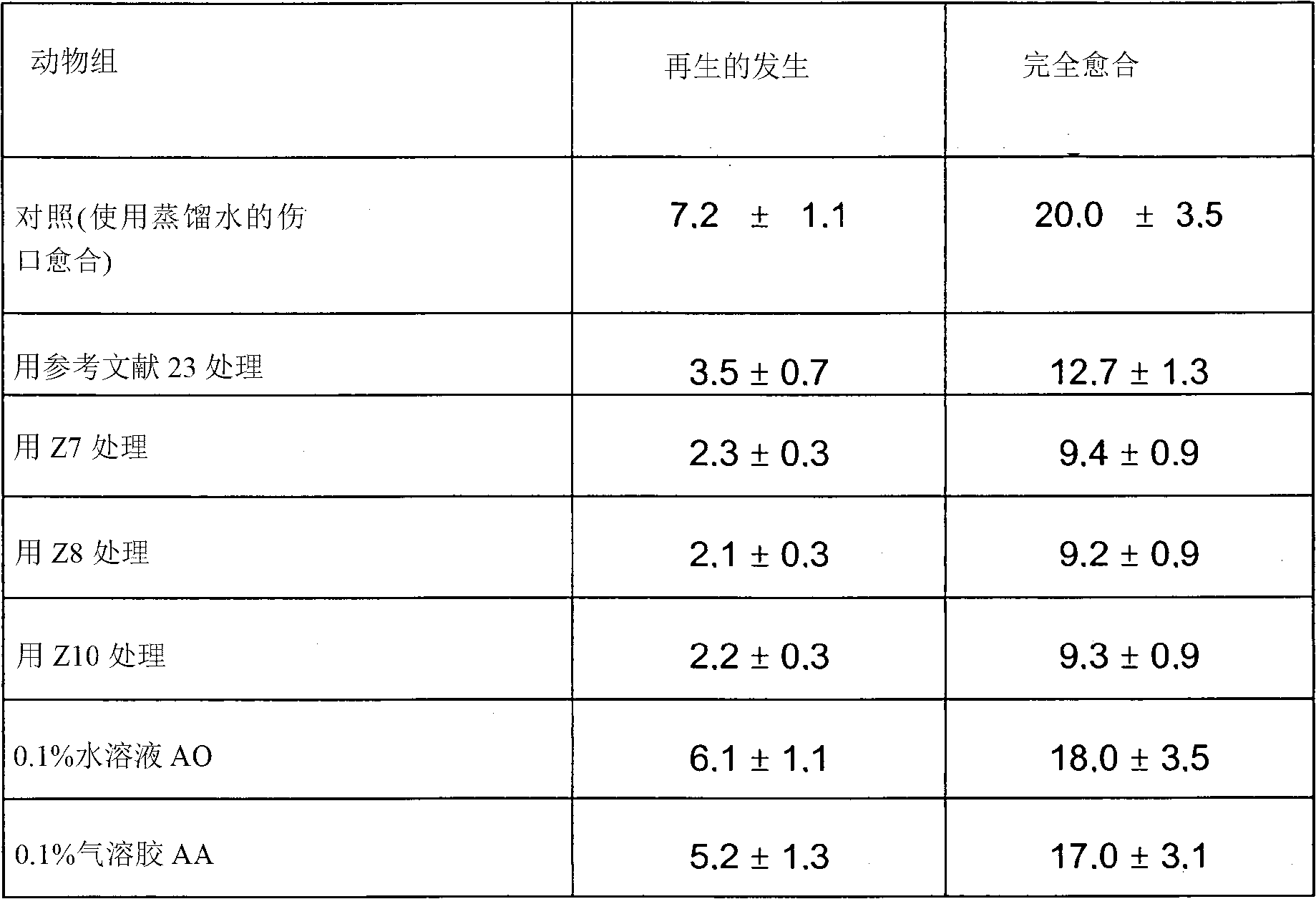
Pharmaceutical comprising myramistin
The invention relates to a pharmaceutical including benzyldimethyl(3-[myristoylamino]propyl)ammonium chloride in the monohydrate form or in unhydrated form. The pharmaceutical further comprises dimethyl(3-[myristoylamino]propyl)ammonium oxide and / or dimethyl(3-[myristoylamino]propyl)amine in the appropriate pharmaceutical solvent.
Owner:MEGAINPHARM
Prosoma liposome preparation, its production method and using method
ActiveCN1698585AFix stability issuesSimple manufacturing processLiposomal deliverySolubilityActive agent
Disclosed is a prosoma liposome preparation, its production method and using method, wherein the preparation comprises medicament, solvent, phosphatide and adjunct, the solvent is non-aqueous solvent with hydrophilous nature, the adjunct is surface active agent. The solvent can be non-aqueous solvent having hydrophilous nature and good dissolving property to medicament, phosphatide and adjunct. the surface active solvent can be anionic or non-ionic surface active agent. The production method consists of dissolving the medicament with solvent, charging phosphatide and adjunct, fully dissolving so as o obtain the end product.
Owner:新疆维吾尔自治区包虫病临床研究所 +2
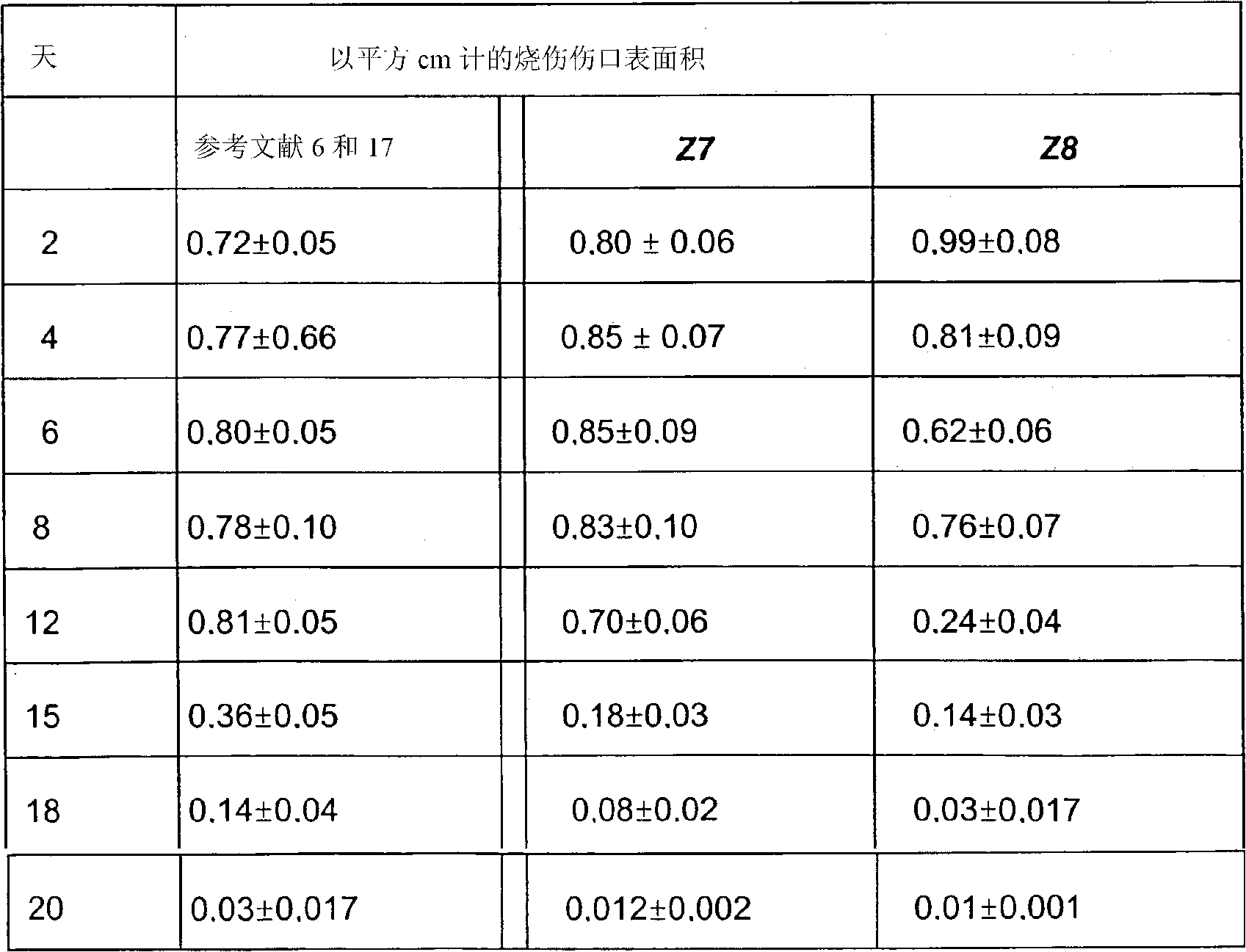
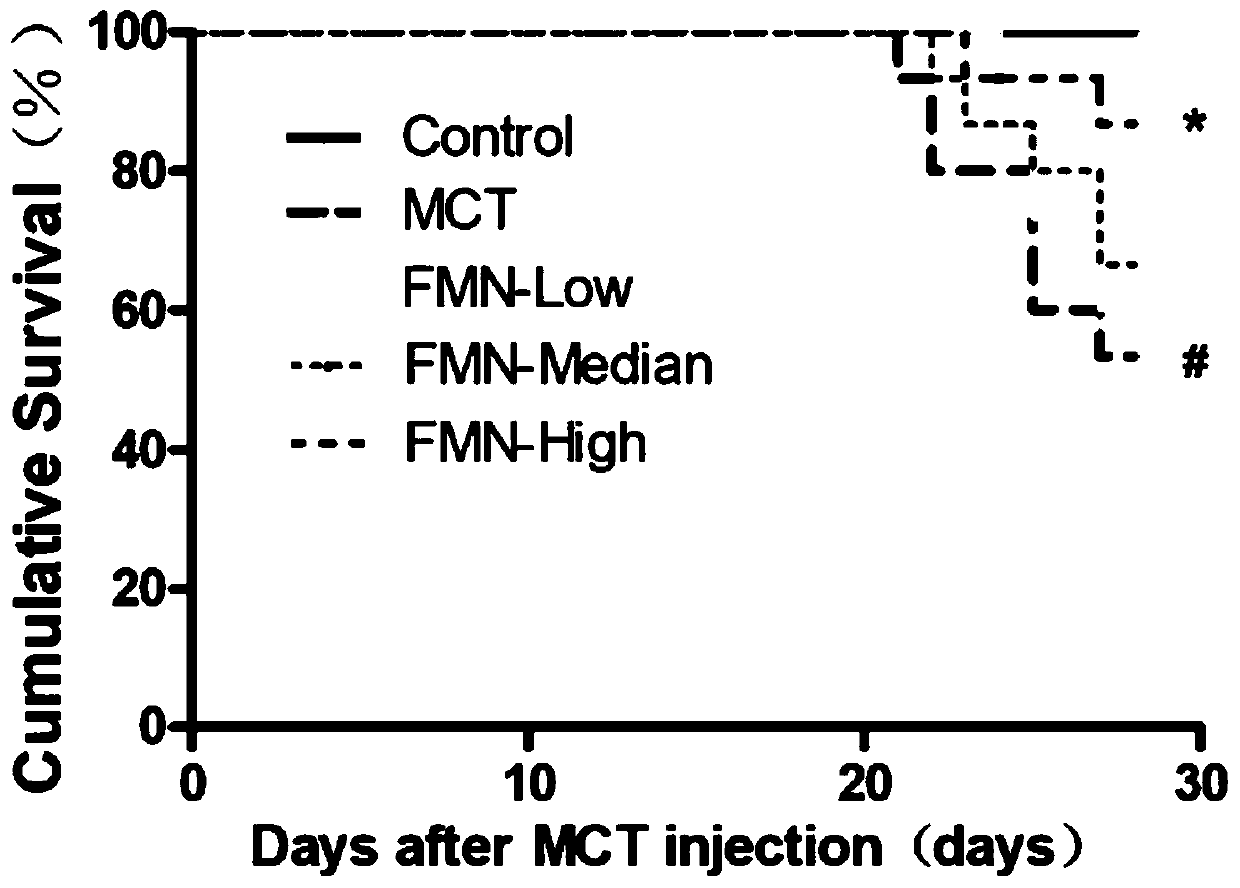
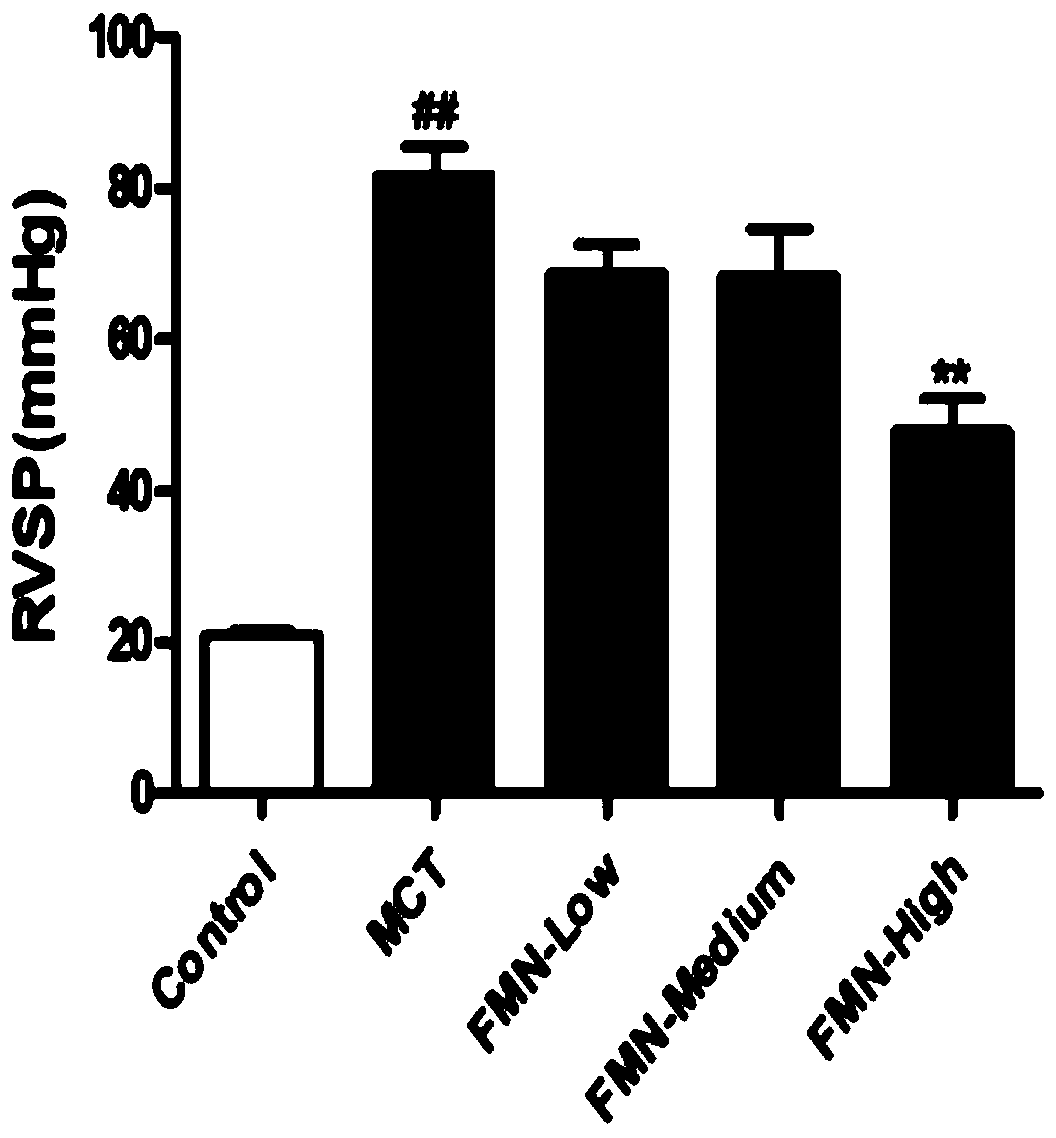
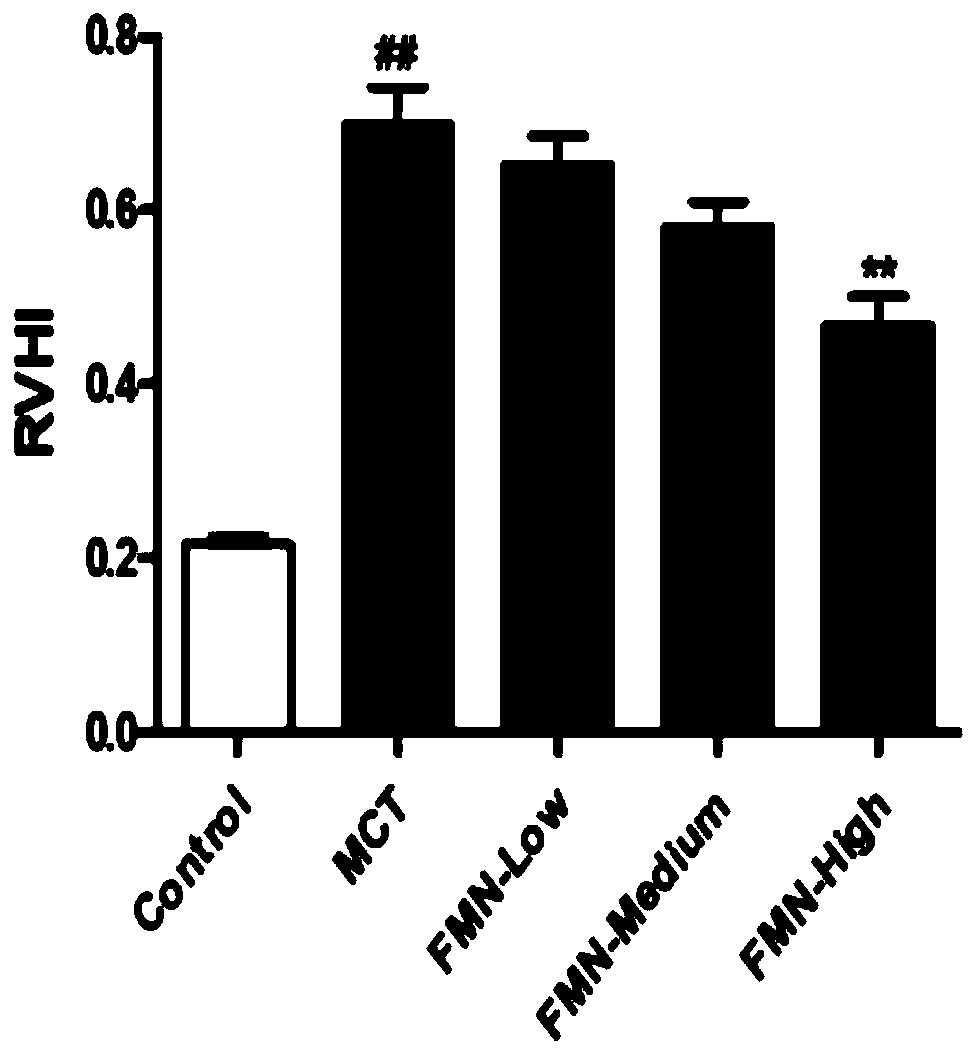
Application of formononetin to preparation of drugs for treating pulmonary hypertension and drugs for treating pulmonary hypertension
InactiveCN110141565APromote hypertrophyProtect heart functionOrganic active ingredientsRespiratory disorderArterial smooth muscle cellsApoptosis
The invention discloses application of formononetin to preparation of drugs for treating pulmonary hypertension and the drugs for treating pulmonary hypertension. The pulmonary hypertension drugs areoral drugs or injection drugs. The pulmonary hypertension drugs in the form of injection drugs are obtained by dissolving the formononetin in pharmaceutical solvents. The pharmaceutical solvents comprise dimethyl sulfoxide and / or olive oil. According to the present invention, the proliferation of pulmonary artery smooth muscle cells can be inhibited and the apoptosis of the pulmonary artery smoothmuscle cells can be promoted by the formononetin, pulmonary vascular remodeling can be significantly improved, pulmonary vascular resistance and pulmonary artery pressure can be reduced, the formononetin plays a role in reducing blood pressure stably, pulmonary function and right heart function can be restored, human impaired function can be normalized and complications can be treated as a whole.
Owner:LISHUI CENT HOSPITAL
Pharmaceutical composition overcoming postoperative sleep disorder
InactiveCN105287518ATotal sleep time increasedImprove subjective sleep qualityOrganic active ingredientsNervous disorderSleeping disordersNarcotic
The invention relates to the field of pharmaceutical compositions, and especially relates to a pharmaceutical composition overcoming postoperative sleep disorder. The provided pharmaceutical composition contains a pharmaceutical solvent carrier and dexmedetomidine or pharmaceutically-acceptable salts thereof dissolved in the solvent carrier. The pharmaceutical composition is used to improve postoperative patient sleep structure, thereby improving patient sleep quality. The pharmaceutical composition also is capable of mitigating patient pain, is safe to use and low in cost, and is usable by being matched with one or more of other sedatives, narcotics, hypnotics and opioids.
Owner:PEKING UNIV FIRST HOSPITAL
A kind of β2 receptor agonist inhalation aerosol and products containing the inhalation aerosol
ActiveCN110840864BEfficient depositionImprove stabilityPowder deliveryOrganic active ingredientsAlkanePolyethylene glycol
The invention provides a β2 receptor agonist inhalation aerosol and products containing the inhalation aerosol. The β2 receptor agonist inhalation aerosol of the present invention is composed of active ingredient formoterol or its pharmaceutically acceptable salt, pharmaceutical carrier, pharmaceutical solvent, polyethylene glycol, povidone and hydrofluoroalkane propellant Composition, by adding the appropriate auxiliary material povidone into the product prescription, and strictly limiting the content of each component in the prescription, and using a combination of fluorocarbon-coated metal cans and quantitative metal valves as the container system; the product stability and effectiveness are improved. Pulmonary deposition has obvious advantages and good market prospects.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD +1
Preparation method of crystalized drug coating on surface of coronary balloon
The invention provides a preparation method of a crystalized drug coating on the surface of a coronary balloon, and belongs to the technical field of drug loading. The preparation method comprises the following steps: (1) mixing acetone and isobutanol to obtain a drug solvent; (2) dissolving a loaded drug in a drug solvent, adding ethanol, and carrying out ultrasonic treatment to obtain a drug dip-coating solution; (3) blowing the coronary balloon for later use; (4) immersing the coronary balloon in the medicine dip-coating solution to obtain the coronary balloon coated with the medicine; and (6) loading the coronary balloon into a negative pressure device, crystallizing, and taking out to obtain the coronary balloon loaded with the crystal drug coating. The medicine is combined on the surface of the coronary balloon in a dip-coating mode, the medicine on the surface of the coronary balloon is subjected to crystallization growth through vacuum operation, the obtained medicine layer on the surface of the coronary balloon is uniform and free of stripping, the balloon is not exposed, crystals are fine, and the medicine loading capacity is stable.
Owner:HANGZHOU BARTY MEDICAL TECH CO LTD
A kind of sodium alginate-based pH-responsive drug microcapsules and preparation method thereof
InactiveCN105078924BGood pH responsivenessGood mechanical properties of wall materialOrganic active ingredientsPharmaceutical non-active ingredientsOil phaseDouble bond
Owner:HEBEI UNIVERSITY
Patch for treating rheumatic arthritis and preparation method thereof
InactiveCN102670624BIntestinal first pass effectWith sustained release and long-actingOrganic active ingredientsAntipyreticDiseasePharmacologic action
The invention discloses a patch for treating rheumatic arthritis and a preparation method thereof. The patch consists of a main medicament for treating rheumatic arthritis, a medicament solvent, a substrate and a penetrating agent, wherein the main medicament consists of triptolide and lidocaine serving as a pain-relieving medicament for relieving rheumatic arthritis pain. The patch is medical controlled-release slow release preparation, has a comprehensive pharmacological action, is used for blocking major development pathologic loops of the rheumatic arthritis disease, and has high specificity and a lasting pharmacological effect; and the preparation method is simple.
Owner:FUJIAN ACAD OF MEDICAL SCI
A kind of terlipressin acetate preparation and preparation method thereof
ActiveCN110251469BPromote precipitationEasy to shapePowder deliveryPeptide/protein ingredientsDrugs solutionGlycine
The invention relates to the technical field of pharmacy, in particular to a terlipressin acetate preparation and a preparation method thereof. The preparation method of the terlipressin acetate preparation comprises the following steps: freezing and drying a drug solution obtained by mixing terlipressin acetate, glycine and a drug solvent to obtain a terlipressin acetate preparation; The weight ratio of terlipressin acetate and glycine is 1:10-20; the pH of the drug solution is 3.5-4.5. The terlipressin acetate preparation obtained by the specific preparation method of the present invention has a complete appearance, which improves the product yield; the water content of the preparation is low, which improves the stability of the preparation during transportation and storage; it can be quickly reconstituted; and Under the conditions of high temperature and light, the impurity content is small, which improves the safety of the preparation in clinical administration.
Owner:南京诺卡医药技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com