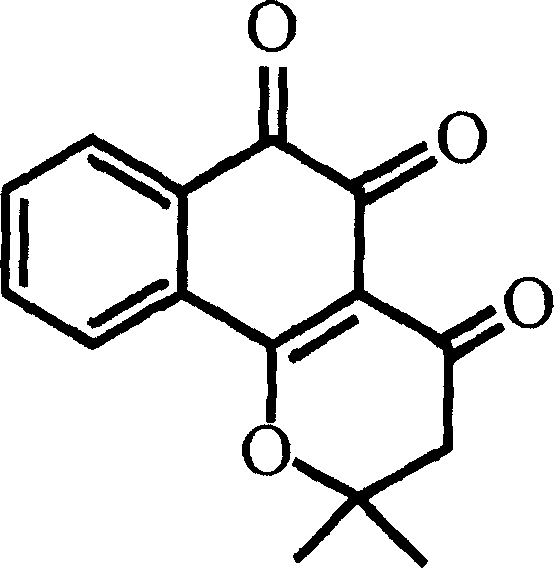
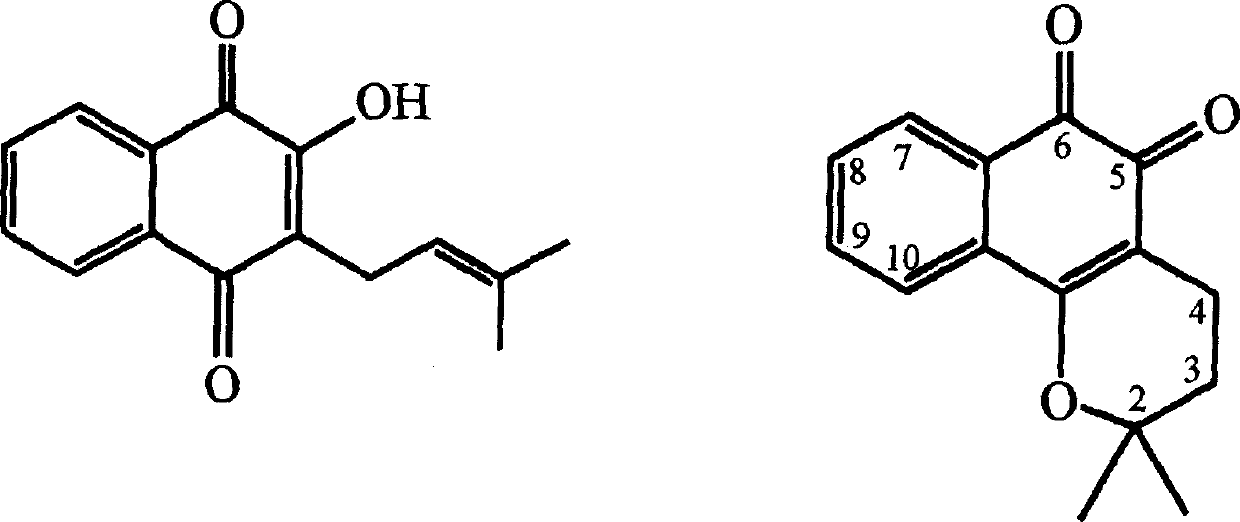
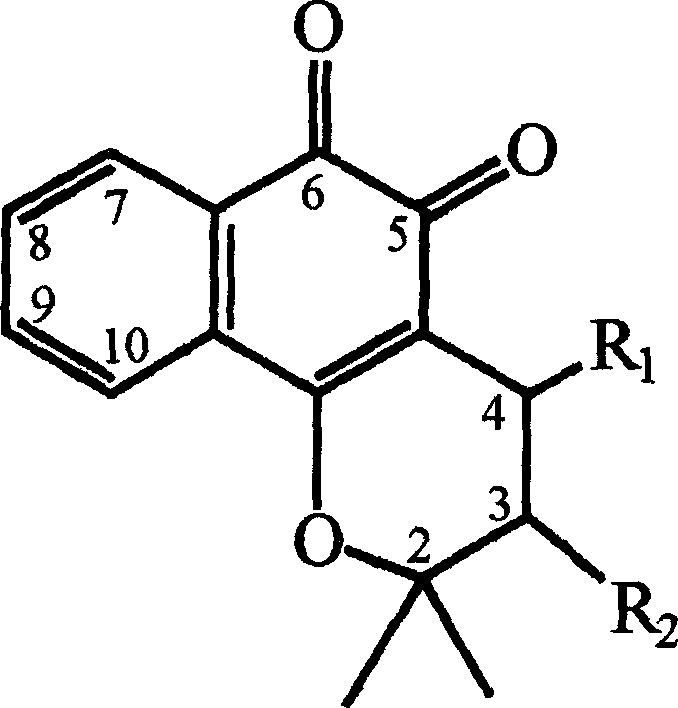
Pharmaceutical compositions containing beta-lapachone, or derivatives or analogs thereof, and methods of using same
A composition and analog technology, which can be applied in the directions of medical preparations containing active ingredients, drug combinations, medical preparations without active ingredients, etc., can solve the problems of low solubility of beta-paradone and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0150] 1. Evaluation of known solvent systems that increase the solubility of hydrophobic pharmaceutical substances and are acceptable
[0151] a. Preparation of β-paradone and hydroxypropyl-β-cyclodextrin (HPBCD) solution
[0152] Various acceptable and well-known pharmaceutical solvent systems were evaluated for increasing the solubility of hydrophobic pharmaceutical substances using β-paradone. As shown in Table 5 below, among the various solutions evaluated, the solutions reached the lowest concentration targeted (10 mg / ml). However, none of these solvent systems could be diluted 5-fold with sterile saline without significant precipitation of β-paradone in solution. In addition, most co-solvents and surfactants have their own toxicity and tolerance issues that need to be addressed during high-dose administration.
[0153] table 5
[0154] Solvent system Undiluted 5 times diluted *
[0155] (mg / ml) (mg / ml)
[0156] Poloxa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com