A kind of terlipressin acetate preparation and preparation method thereof
A technology of terlipressin and acetic acid is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc., and can solve the problems of low product yield, poor stability, limited application and the like, To achieve the effect of improving stability and reconstitution, improving stability and shape, and being easy to industrialize production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] The present invention relates to a method of preparing acetate-specific pressurin preparation, comprising the steps of:
[0016] The drug solution obtained by mixing acetate, glycine, and drug solvent is frozen and dried to obtain a teelectric acid supplement preparation;
[0017] The weight ratio of the vine acid special-absorbent and glycine is 1: 10-20;
[0018] The pH of the drug solution was 3.5-4.5.
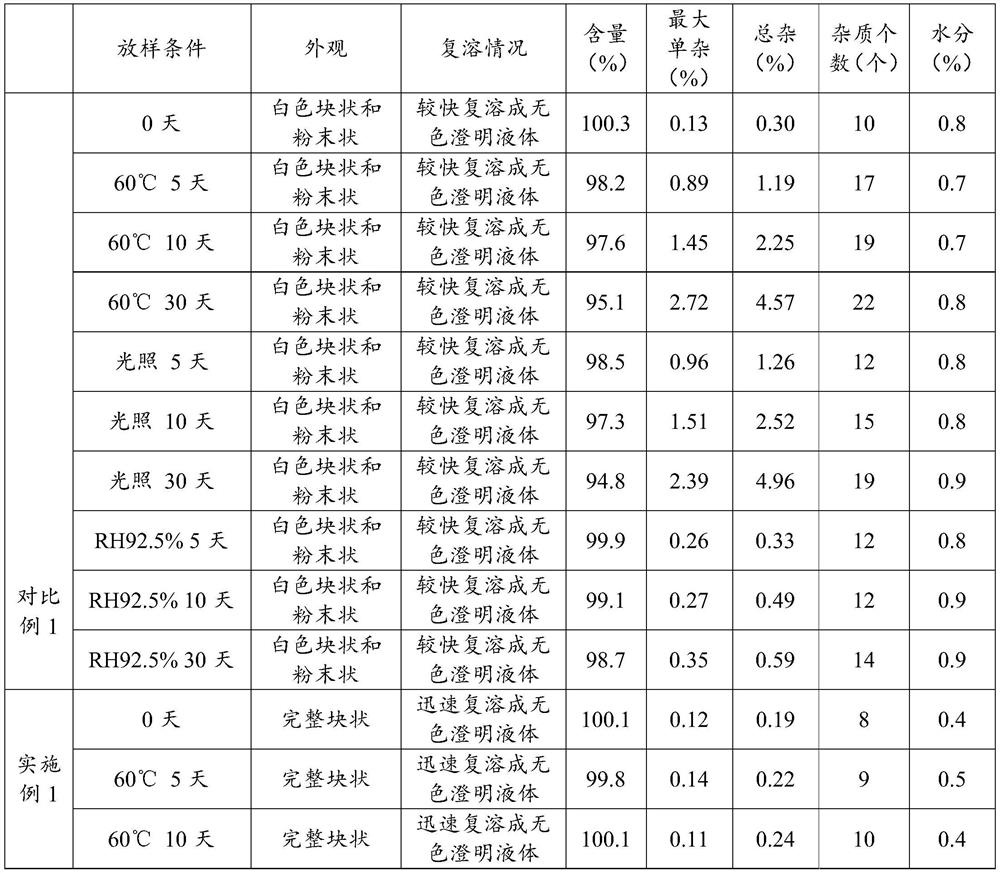
[0019] In the present invention, glycine is used as a carrier, and the glycine has a good compatibility with acetate-specific pressurin, and glycine can be used as an antioxidant, and has a certain antibacterial effect. The use of acetic acid of acetic acid with a weight ratio of 1: 10-20 and glycine in the drug, the drug load is carried on the carrier, more conducive to the precipitation and molding of the drug, thereby improving the stability and shape of the drug. It is conducive to lyophilized drugs to form fluffy structures, thereby improving the solubility and stab...
Embodiment 1
[0072] Having an acetic acid terlipressin preparation of pigment formulations, comprising the steps of:
[0073] (A) amount of water for injection to 40L dosing tank, spare; glycine weighed 938g water for injection was added, stirred and completely dissolved; Terry weighed 62.5g drug vasopressin acetate was added to the glycine solution stirred dissolved; using 1mol / L hydrochloric acid solution was adjusted to pH 4.0, water for injection qs to 50L;
[0074] (B) Intermediate detection: Apply chemical intermediate, intermediate index measured content and the like;
[0075] (C) Filter: using 0.22μm PVDF membrane filter sterilized by filtration;
[0076] (D) Filling: The intermediate liquid detection result, the liquid filling the borosilicate resistant to 7mL vial, using semi-bromobutyl rubber stopper tamponade standby;
[0077] (E) freeze-drying process:
[0078] The vials containing liquid is placed on the partition plate lyophilizer, firstly to cool the separator, and then turn ...
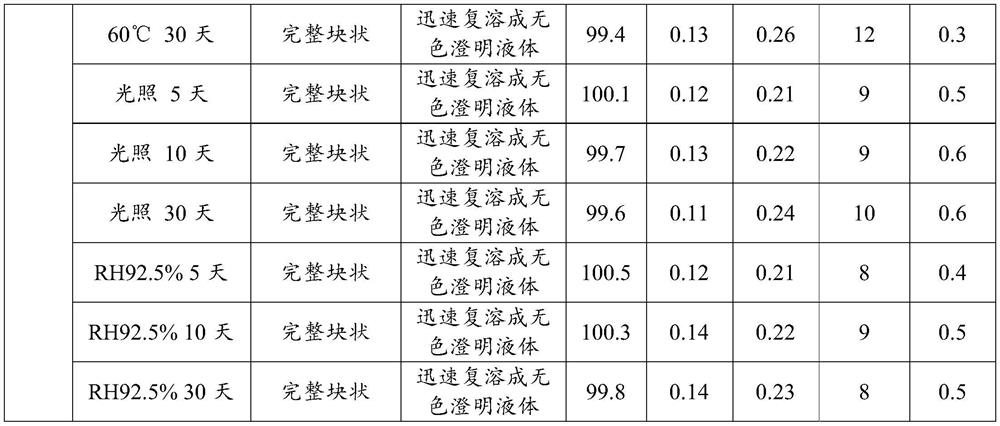
Embodiment 2
[0091] Having an acetic acid terlipressin preparation of pigment formulations, comprising the steps of:
[0092] (A) amount of water for injection to 40L dosing tank, spare; glycine weighed 1000g water for injection was added, stirred and completely dissolved; 50g weighed Terry drug vasopressin acetate was added to the glycine solution, stirred was dissolved; using 1mol / L hydrochloric acid solution was adjusted to pH 3.8, water for injection qs to 50L;
[0093] (B) Intermediate detection: Apply chemical intermediate, intermediate index measured content and the like;
[0094] (C) Filter: using 0.22μm PVDF membrane filter sterilized by filtration;
[0095] (D) Filling: The intermediate liquid detection result, the liquid filling the borosilicate resistant to 7mL vial, using semi-bromobutyl rubber stopper tamponade standby;
[0096] (E) freeze-drying process:
[0097] The vials containing liquid is placed on the partition plate lyophilizer, firstly to cool the separator, and then t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com