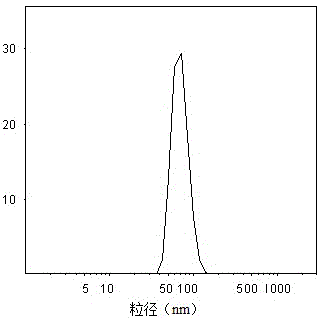
Paclitaxel lipid nanoparticle injection liquid with anti-tumor activity
A technology of lipid nanoparticles and anti-tumor activity, which is applied in anti-tumor drugs, liquid delivery, emulsion delivery, etc. It can solve the problem of drug resistance of tumor cells to chemotherapeutic drugs, limit SLN drug loading capacity, and multiple drug release curves Changes and other issues to achieve the effect of overcoming tumor drug resistance, excellent tumor drug resistance, and avoiding allergic reactions or toxic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
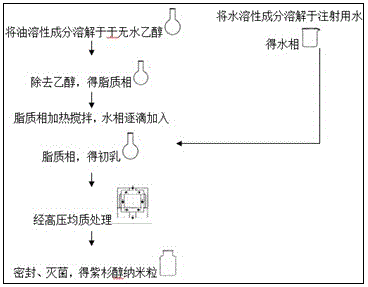
[0063] The prescription and preparation technology of embodiment 1 paclitaxel preparation
[0064] Prescription 1:
[0065]Paclitaxel 1 %
[0066] Oleic acid 0.8%
[0067] Phospholipids 2 %
[0068] Vitamin E 5%
[0069] Cholesterol 1 %
[0070] DSPE-PEG2000 0.5%
[0071] Sucrose 10%
[0072] EDTA0.5%
[0073] The rest is water for injection.
[0074] Preparation method 1:
[0075] (1) Dissolve the prescribed amount of vitamin E, phospholipids, oleic acid, cholesterol, DSPE-PEG2000, and paclitaxel in an appropriate amount of absolute ethanol, and remove the ethanol by rotary evaporation under reduced pressure to obtain the lipid phase;
[0076] (2) Mix sucrose, EDTA, and water for injection, and stir magnetically until each part is dissolved to obtain the aqueous phase;
[0077] (3) The lipid phase was heated and stirred at 60°C, the water phase was added dropwise to the lipid phase, and stirred rapidly for 10 minutes to obtain milky white colostrum;
[0078] (4) Pl...
Embodiment 2
[0085] Prescription 2:
[0086] Paclitaxel 0.5%
[0087] Oleic acid 0.65%
[0088] Lecithin 2.6%
[0089] Vitamin E 6 %
[0090] DSPE-PEG2000 1%
[0091] Sucrose 10.4%
[0092] Sodium cholate 0.7%
[0093] EDTA0.1%
[0094] The rest is water for injection.
[0095] Preparation method 2:
[0096] (1) Dissolve the prescribed amount of vitamin E, lecithin, oleic acid, paclitaxel, and DSPE-PEG2000 in an appropriate amount of absolute ethanol, and remove the ethanol by rotary evaporation under reduced pressure to obtain the lipid phase;
[0097] (2) Mix sucrose, sodium cholate, and EDTA with water for injection, and stir magnetically until each part is dissolved to obtain an aqueous phase;
[0098] (3) The lipid phase was heated and stirred at 60°C, the water phase was added dropwise to the oil phase, and stirred rapidly for 10 minutes to obtain milky white colostrum;
[0099] (4) Place the colostrum in a high-pressure milk homogenizer, homogenize it with a pressure of 11...
Embodiment 3
[0101] Prescription 3:
[0102] Paclitaxel 3 %
[0103] Oleic Acid 2.4%
[0104] Lecithin 4 %
[0105] Vitamin E 15%
[0106] Cholesterol 1.2%
[0107] DSPE-PEG2000 1%
[0108] Sucrose 10.05%
[0109] Sodium cholate 0.7%
[0110] EDTA0.5%
[0111] The rest is water for injection.
[0112]
[0113] Preparation method 3:
[0114] (1) Dissolve the prescribed amount of vitamin E, lecithin, oleic acid, paclitaxel, cholesterol, and DSPE-PEG2000 in an appropriate amount of absolute ethanol, and remove the ethanol by rotary evaporation under reduced pressure to obtain the lipid phase;
[0115] (2) Mix sucrose, sodium cholate, and EDTA with water for injection, and stir magnetically until each part is dissolved to obtain an aqueous phase;
[0116] (3) The lipid phase was heated and stirred at 60°C, the water phase was added dropwise to the oil phase, and stirred rapidly for 10 minutes to obtain milky white colostrum;
[0117] (4) Place the colostrum in a high-pressure milk ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com