Mini-pill type nicergoline capsule and preparation method thereof
A technology of nicergoline and pellets, applied in the field of medicine, can solve the problems of insufficient release of active ingredients, low absolute bioavailability, complicated production process, etc., and achieves improving bioavailability, avoiding photodecomposition of drugs, and increasing utilization. area effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
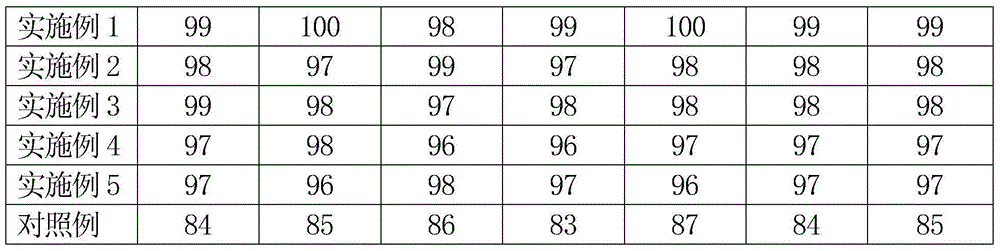
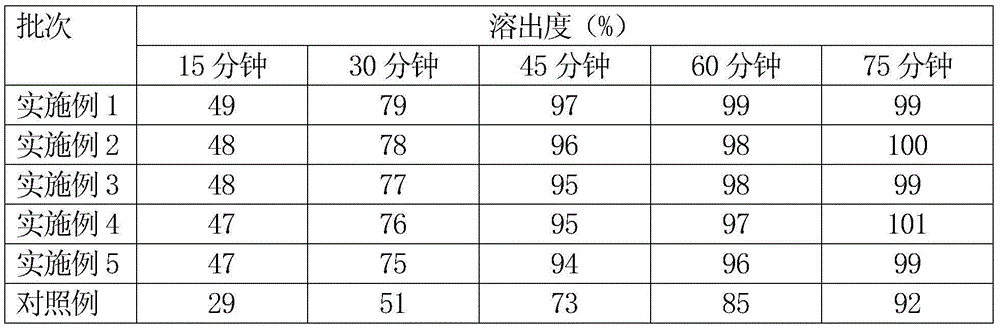
[0026] A micropill type nicergoline capsule is a sustained-release preparation. The micropill type nicergoline capsule is composed of a capsule shell and nicergoline pellets contained in the capsule shell. To the outside, there are blank core, main drug layer and pigment layer. The weight of the main drug layer is 15% of the blank core, and the weight of the pigment layer is 0.5% of the total weight of the blank core and the main drug layer. The diameter of the blank core is 1.2 mm, the particle size of nicergoline pellets is 14-24 mesh, and each capsule contains 15 mg of nicergoline.
[0027] The preparation method of above-mentioned pellet type Nicergoline capsule, comprises the following steps:
[0028] Preparation of main drug layer coating
[0029]Take 400 grams of purified water, slowly add 37.5 grams of hydroxypropyl methylcellulose under stirring, after the dissolution is complete, add 28.1 grams of anhydrous sodium carbonate, 9.4 grams of Tween-80, and 28.1 grams of ...
Embodiment 2
[0034] A micropill type nicergoline capsule is a sustained-release preparation. The micropill type nicergoline capsule is composed of a capsule shell and nicergoline pellets contained in the capsule shell. To the outside, there are blank core, main drug layer and pigment layer. The weight of the main drug layer is 25% of the blank core, and the weight of the pigment layer is 0.7% of the total weight of the blank core and the main drug layer. The diameter of the blank core is 1.1 mm, the particle size of nicergoline pellets is 14-24 mesh, and each capsule contains 20 mg of nicergoline.
[0035] The preparation method of above-mentioned pellet type Nicergoline capsule, comprises the following steps:
[0036] Preparation of main drug layer coating
[0037] Take 600 grams of purified water, slowly add 63.4 grams of hydroxypropyl methylcellulose under stirring, after the dissolution is complete, add 49.3 grams of anhydrous sodium carbonate, 17.6 grams of Tween-80, and 49.3 grams o...
Embodiment 3
[0042] A micropill type nicergoline capsule is a sustained-release preparation. The micropill type nicergoline capsule is composed of a capsule shell and nicergoline pellets contained in the capsule shell. To the outside, there are blank core, main drug layer and pigment layer. The weight of the main drug layer is 40% of the blank core, and the weight of the pigment layer is 0.8% of the total weight of the blank core and the main drug layer. The diameter of the blank core is 1.0 mm, the particle size of nicergoline pellets is 14-24 mesh, and each capsule contains 25 mg of nicergoline.
[0043] The preparation method of above-mentioned pellet type Nicergoline capsule, comprises the following steps:
[0044] Preparation of main drug layer coating
[0045] Take 1000 grams of purified water, slowly add 103 grams of hydroxypropyl methylcellulose under stirring, after the dissolution is complete, add 82 grams of anhydrous sodium carbonate, 30.8 grams of Tween-80, and 82 grams of do...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com