Enveloping process for micro-capsule animal medicament
A capsule-type, animal-based technology, applied in the direction of microcapsules, drug delivery, capsule delivery, etc., can solve the problems of large production volume, high production cost, and large energy consumption of animal drugs, and achieve improved drug bioavailability and product coating The effect of simple process and low production cost requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
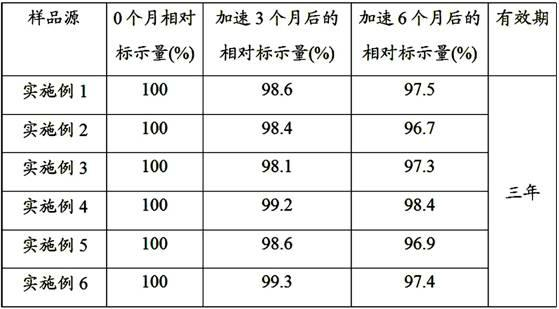
Examples
Embodiment 1
[0048] Embodiment 1: Production formula and process of sour nimulin moisture-proof microcapsules
[0049] 1.1 Capsule core drug powder formula: by weight
[0050] 10 parts of warnemulin hydrochloride
[0051]Microcrystalline cellulose: 30 parts
[0052] Starch: 25 parts
[0054] 1.2 Coating powder: in terms of weight percentage, it is composed of 98% talcum powder and 2% HPMC; the dosage accounts for 5% of the weight of the capsule core medicine powder;
[0055] 1.3 Polishing powder: the dosage accounts for 2% of the total weight of capsule core medicine powder;
[0056] 1.4 Purified water: 70 parts according to the measurement standard of capsule core drug powder formula;
[0057] Step 1: Weigh the material
[0058] According to the above formula, weigh each component in a three-dimensional mixer and mix for 30 minutes for later use, and weigh purified water, coating powder, and polishing powder for later use;
[0059] The second st...
Embodiment 2
[0074] Embodiment 2: Production formula and process of sour nimulin moisture-proof microcapsules
[0075] 1.1 Capsule core drug powder formula: by weight
[0076] 15 parts of warnemulin hydrochloride
[0077] Microcrystalline cellulose: 35 parts
[0078] Starch: 30 parts
[0079] Talcum powder: 30 parts;
[0080] 1.2 Coating powder: by weight percentage, it is composed of 92% talcum powder and 8% HPMC; the dosage is 8% of the total weight of capsule core drug powder;
[0081] 1.3 Polishing powder: the dosage is 2% of the total weight of capsule core drug powder;
[0082] 1.4 Purified water: 75 parts according to the measurement standard of capsule core drug powder formula;
[0083] Step 1: Weigh the material
[0084] According to the above formula, weigh each component in a three-dimensional mixer and mix for 30 minutes for later use, and weigh purified water, coating powder, and polishing powder for later use;
[0085] The second step: making soft material
[00...
Embodiment 3
[0100] Example 3: Production formula and process of taste-masking microcapsules enrofloxacin
[0101] 1.1 Capsule core drug powder formula: 50 parts by weight of enrofloxacin, 40 parts of microcrystalline cellulose;
[0102] 1.2 Coating powder: by weight percentage, it is composed of 90% 200-mesh monoglyceride stearate superfine powder, 5% HPMC, and 5% sweetener; the dosage accounts for 8% of the total weight of the capsule core medicine powder;
[0103] 1.3 Polishing powder: the dosage accounts for 2% of the total weight of capsule core medicine powder;
[0104] 1.4 Purified water: 60 parts according to the measurement standard of capsule core medicine powder formula;
[0105] Step 1: Weigh the material
[0106] According to the above formula, weigh each component in a three-dimensional mixer and mix for 30 minutes for later use, and weigh purified water, coating powder, and polishing powder for later use;
[0107] The second step: making soft material
[0108] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com