Sustained-release micro-pellet of trimetazidine and preparation process thereof
A technology of trimetazidine and sustained-release pellets, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Problems such as short concentration maintenance time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 Preparation of pill core containing medicine
[0067] (1) Prescription:
[0068] Trimetazidine Hydrochloride 250g
[0069] Microcrystalline Cellulose 750g
[0070] 50% ethanol appropriate amount
[0071] (2) Preparation process:
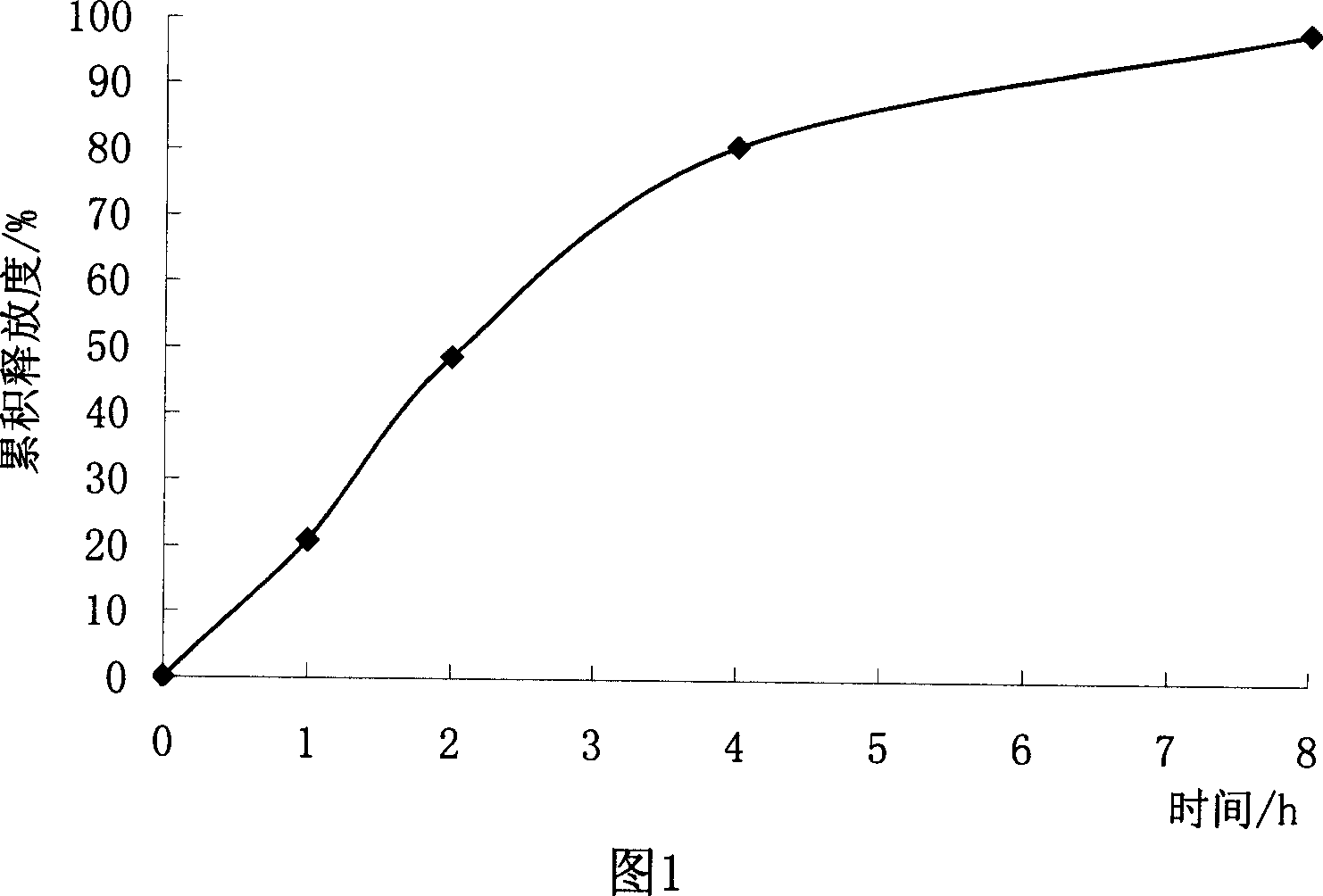
[0072] Get trimetazidine crude drug, pulverize and pass through a 100-mesh sieve, add microcrystalline cellulose, and fully mix; add an appropriate amount of wetting agent (such as 50% ethanol) to the mixed material to make soft material; Put it into the extruder and extrude it into a strip-shaped extrudate; quickly add the extrudate to the spheronizer for spheronizing, and take it out after a certain period of time to make pellets; bake the prepared pellets at 50°C for 3~ After 6 hours, dry pellets were obtained. Results The micropills were smooth and spherical in appearance. At this time, capsules could be filled directly or film-coated with water-soluble coating materials to obtain immediate-release micropills.
Embodiment 2
[0074] Coat the pellet core (that is, uncoated immediate-release pellets) prepared in Example 1 with Kollicoat SR 30D to obtain trimetazidine sustained-release pellets.
[0075] (1) Prescription of coating solution:
[0076] Kollicoat SR 30D 100g
[0077] Propylene glycol 3g
[0079] water 250g
[0080] (2) Preparation process:
[0081] Mix Kollicoat SR 30D and water evenly, add propylene glycol and talcum powder, stir at high speed and homogenize to obtain the coating solution. Using low-spray micro-fluidized bed equipment, add 120 g of uncoated trimetazidine immediate-release pellets of 18 to 24 meshes into the fluidization chamber, use a spray gun with a nozzle diameter of 1 mm, and adjust the fan frequency converter to make the blast flow approx. 125L·min-1, so that most of the pellets are blown up 7-12cm in the fluidization chamber, the atomizing gas pressure is adjusted to 0.2Mpa, and the flow rate of the constant flow pump is 0.9-1.2mL·min-...
Embodiment 3
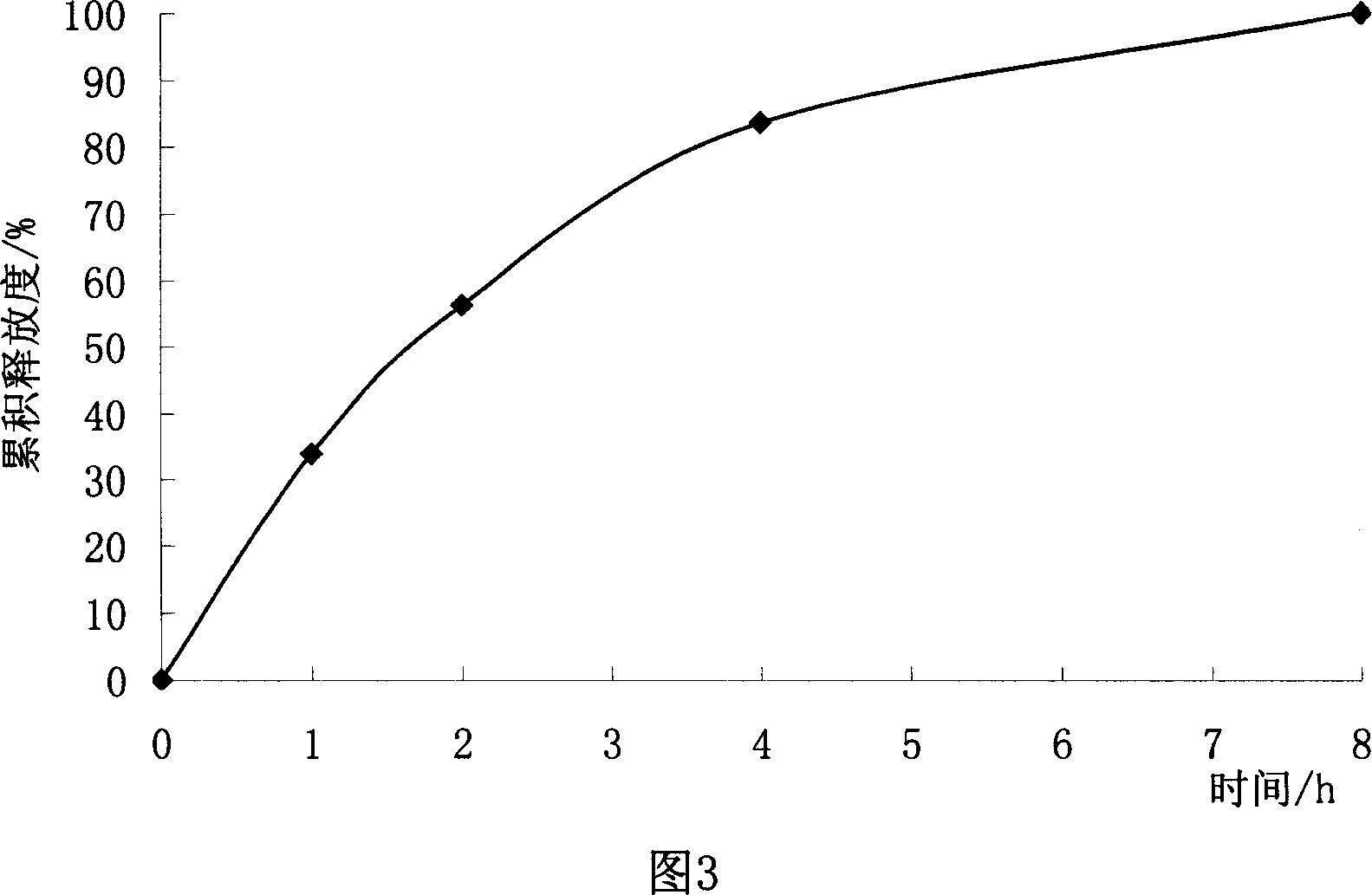
[0084] The drug-containing pellet cores (ie uncoated immediate-release pellets) prepared in Example 1 were coated according to the following coating solution prescription to obtain trimetazidine sustained-release pellets.
[0085] (1) Prescription of coating solution:
[0086] Eudragit NE 30D 100g
[0087] 5% HPMC solution 8mL
[0089] water 110g
[0090] (2) Preparation process:
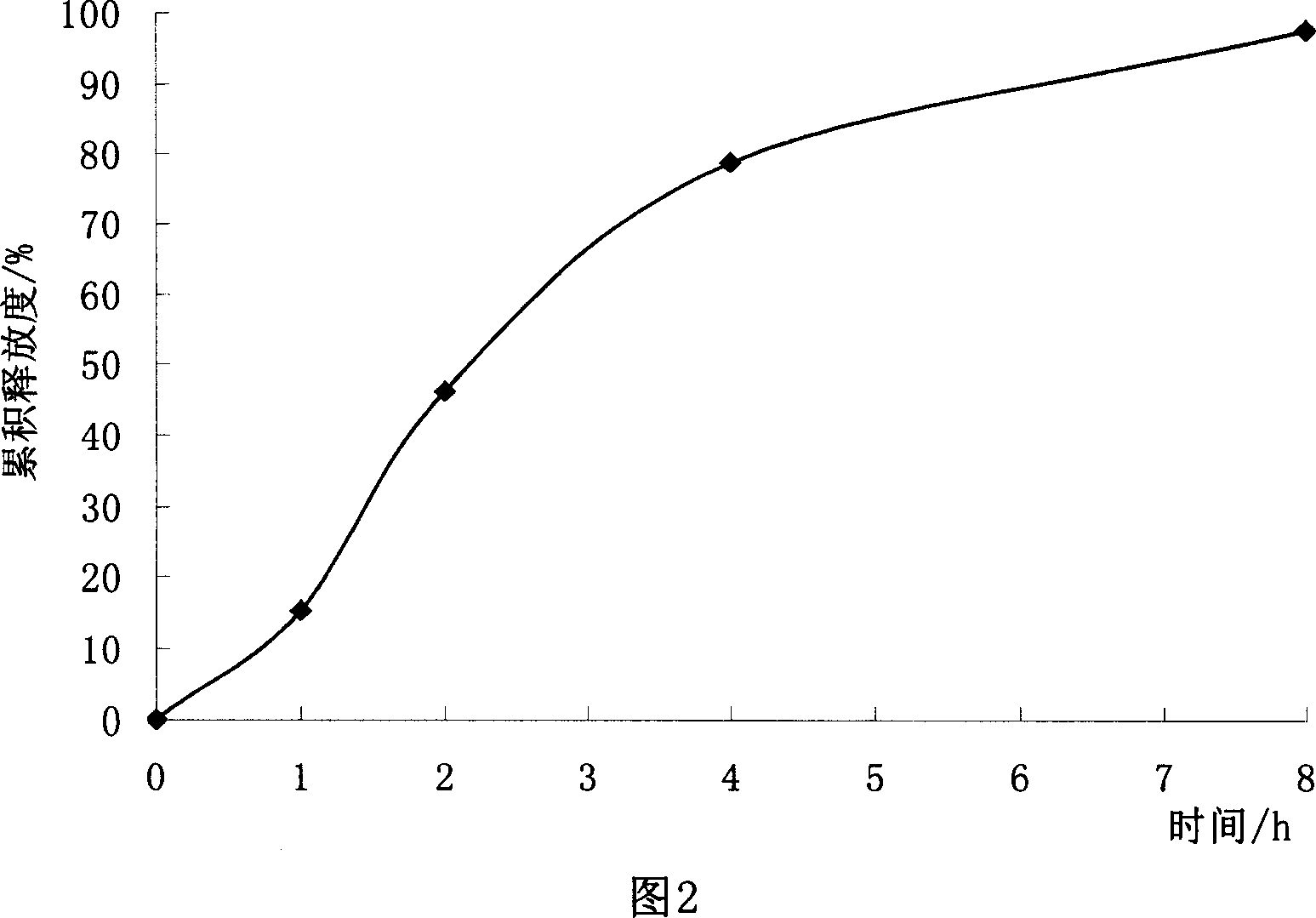
[0091] Take Eudragit NE 30D, HPMC and talcum powder according to the above prescription, homogenize at a high speed, and then coat. After the clothes are finished, put them in a constant temperature oven at 40°C and dry them for 6 hours. 150 mg of coated pellets (equivalent to 35 mg of trimetazidine hydrochloride) was installed in each capsule, and the release rate was measured by the method described in Example 2, and the results are shown in Figure 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com