Atenolol non-pH-dependent sustained release pellets and preparation method thereof
A technology of sustained-release micropills and micropills, which is applied in the direction of bulk delivery, cardiovascular system diseases, drug combination, etc., and can solve the problems of complex preparation process and easy adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
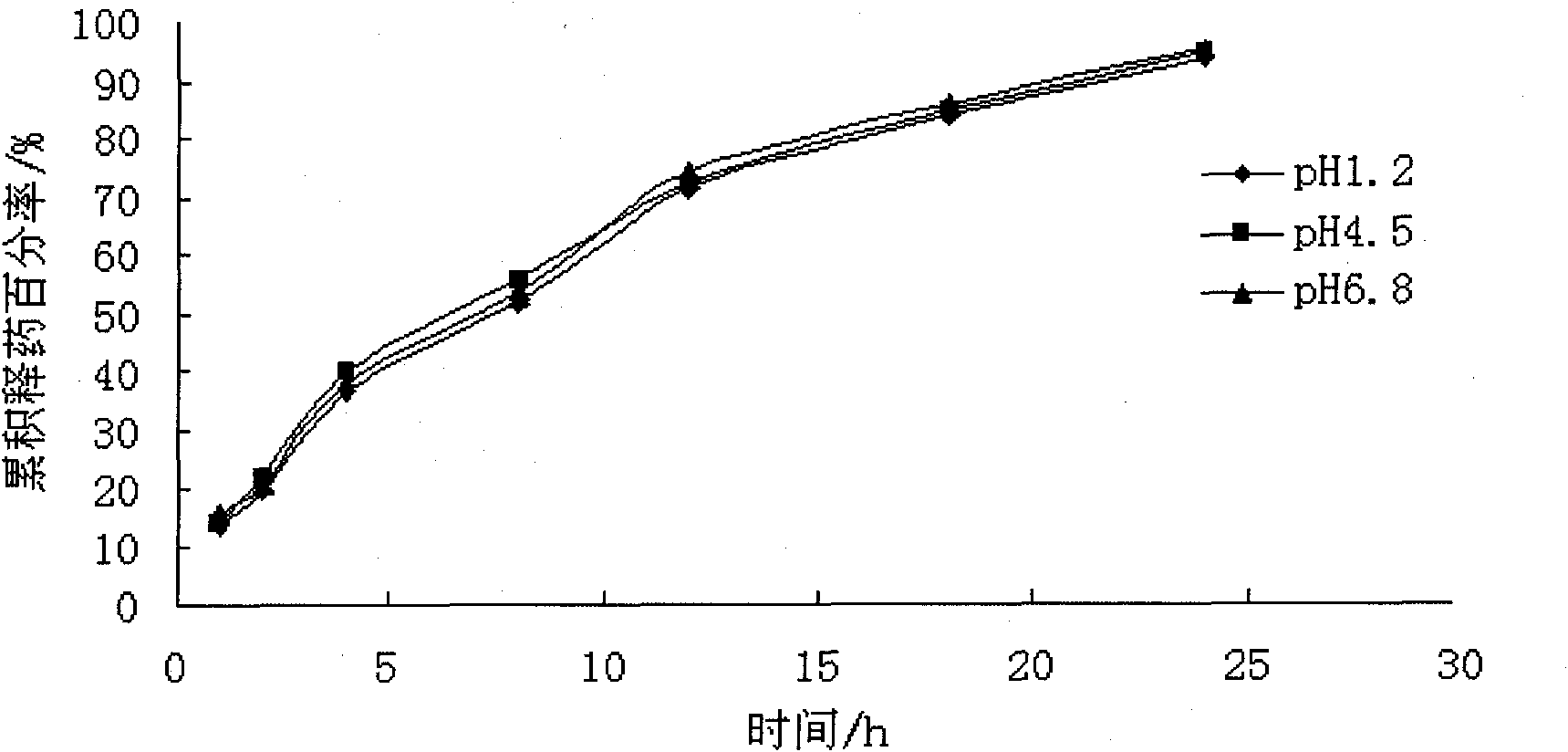
Embodiment 1
[0045] The dosage of each auxiliary material in every 1000 grams of atenolol non-pH dependent sustained-release pellet preparation:
[0046] Microcrystalline Cellulose 590g
[0047] Adipic acid 150g
[0048] Atenolol 250g
[0049] Crospovidone 10g
[0050] Binder: 4% PVP-K30 aqueous solution
[0051] Isolation layer coating material: 5% Opadry 75% ethanol solution (Shanghai Colorcon Company)
[0052] Sustained-release coating layer material: 10% Surelease water dispersion (Shanghai Colorcon Company)
[0053] Preparation of pill core: Pass microcrystalline cellulose, adipic acid, atenolol, and crospovidone through a 100-mesh sieve, mix well, add binder to make soft material, extrude at about 25 rpm, and roll into a ball The speed is about 1500rpm, the ball is spheronized for about 8 minutes, and it is dried in a constant temperature box at 40°C. The yield of the 20-40 mesh pellet core is about 75%.
[0054] Coating: Put 20-40 mesh pill cores in the fluidized bed, coat the...
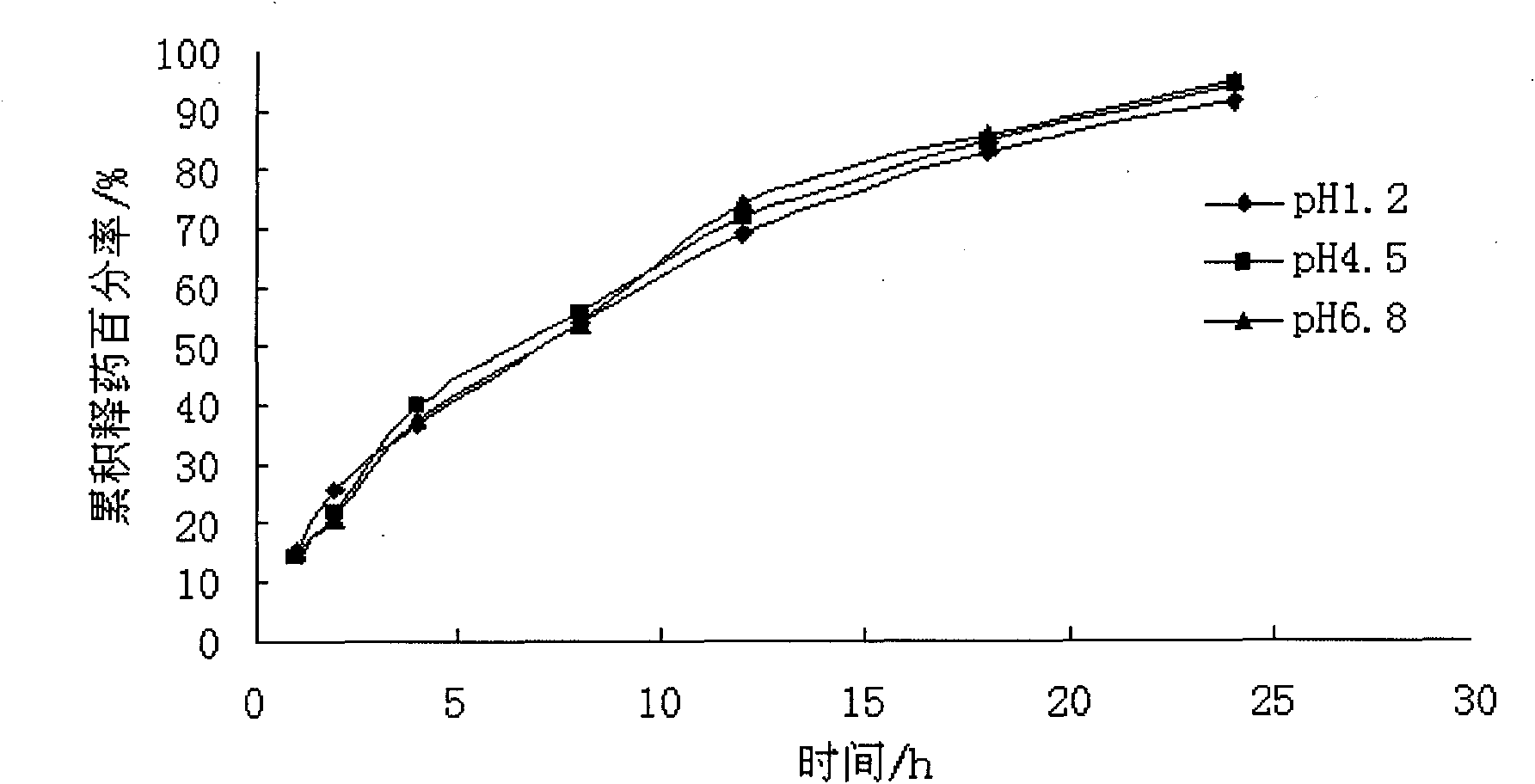
Embodiment 2
[0056] The dosage of each auxiliary material in every 1000 grams of atenolol non-pH dependent sustained-release pellet preparation:
[0057] Microcrystalline Cellulose 640g
[0058] Fumaric acid 100g
[0059] Atenolol 250g
[0060] Sodium carboxymethyl starch 10g
[0061] Binder: 2% HPMC-E5 aqueous solution
[0062] Isolation layer coating material: 4% Opadry 50% ethanol solution (Shanghai Colorcon Company)
[0063] Sustained-release coating layer material: 15% Surelease water dispersion (Shanghai Colorcon Company)
[0064] Preparation of pill core: Pass microcrystalline cellulose, fumaric acid, atenolol, and sodium carboxymethyl starch through a 100-mesh sieve and mix evenly, add binder to make soft material, extrude at about 25 rpm, and roll into a ball The speed is about 1200rpm, rounded for 12 minutes, and placed in a 40°C thermostat to dry to obtain the drug-containing core. The yield of the 20-40 mesh drug-containing core is about 80%.
[0065] Coating: Put 20-40 m...
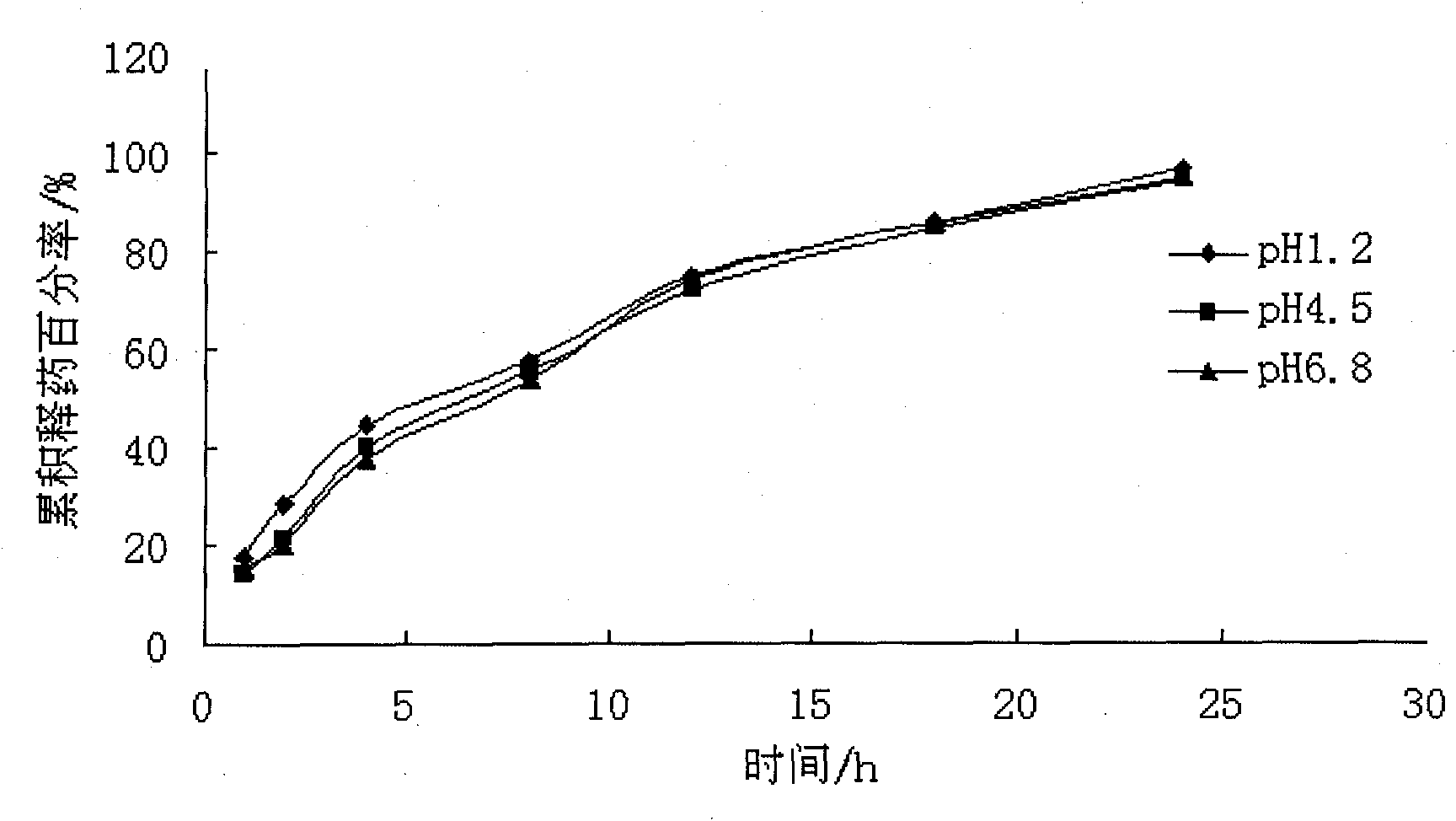
Embodiment 3
[0067] The dosage of each auxiliary material in every 1000 grams of atenolol non-pH dependent sustained-release pellet preparation:
[0068] Starch 400g
[0069] Dextrin 200g
[0070] Adipic acid 150g
[0071] Atenolol 250g
[0072] Adhesive: 25% ethanol aqueous solution Isolation layer Coating material: 80% ethanol solution of 3% HPMC-E5 (containing 15% PEG4000, 20% micropowder silica gel) Sustained release coating layer material: 8% Surelease water dispersion ( Shanghai Colorcon Company)
[0073] Preparation of pill cores: Pass starch, dextrin, adipic acid, and atenolol through a 100-mesh sieve and mix evenly, add binders to make soft materials, extrusion speed 25rpm, spheronization speed 1200rpm, spheronization 9min, set Dried in a constant temperature box at 40°C to obtain the drug-containing core, the yield of the 20-40 mesh drug-containing core is about 75%.
[0074] Coating: Put 20-40 mesh-containing pill cores in the fluidized bed, use 3% HPMC-E5 80% ethanol solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com