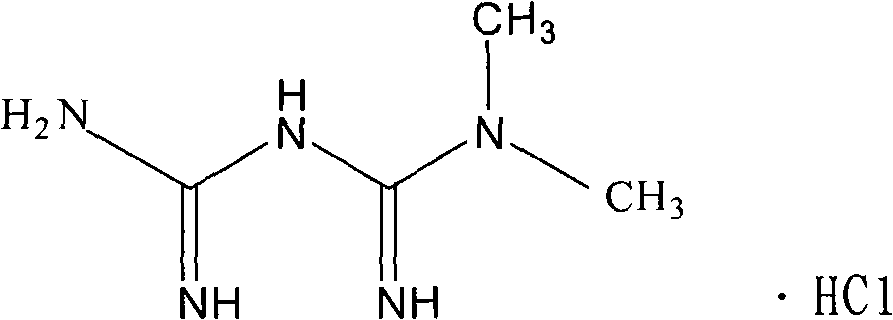
Metformin hydrochloride and Glipizide sustained-release pellet and method of preparing the same
A technology of metformin hydrochloride glipizide and metformin hydrochloride, applied in the field of medicine, can solve problems such as lowering blood sugar, and achieve the effects of reducing blood sugar lowering, reducing the frequency of taking medicines, and reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
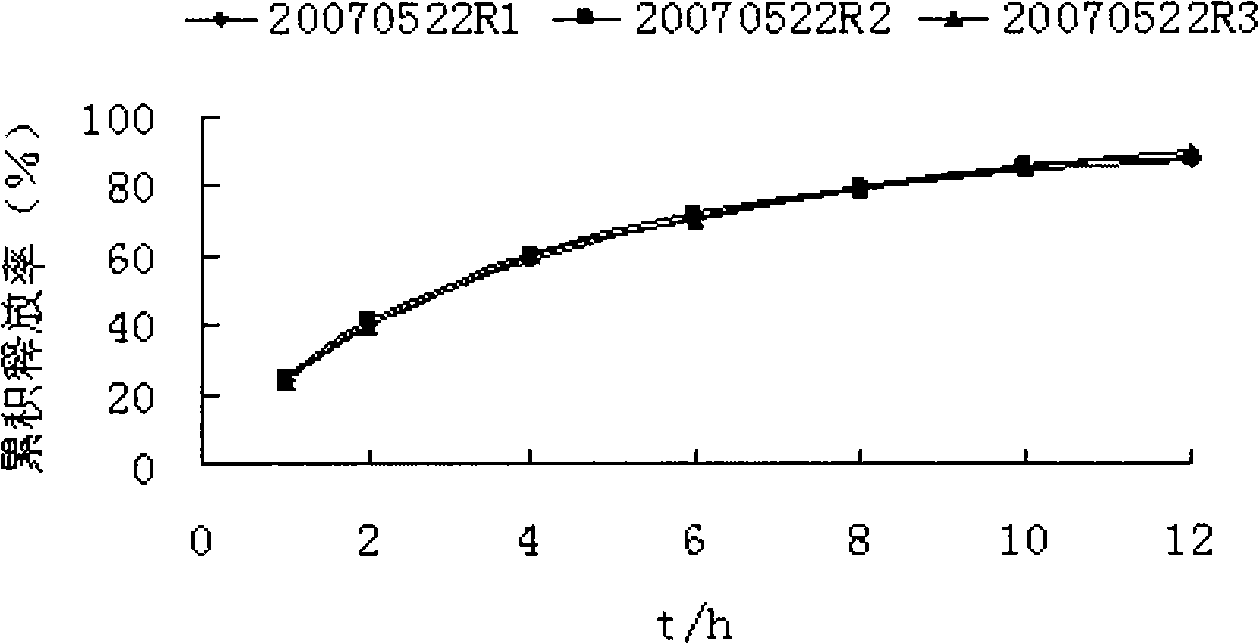
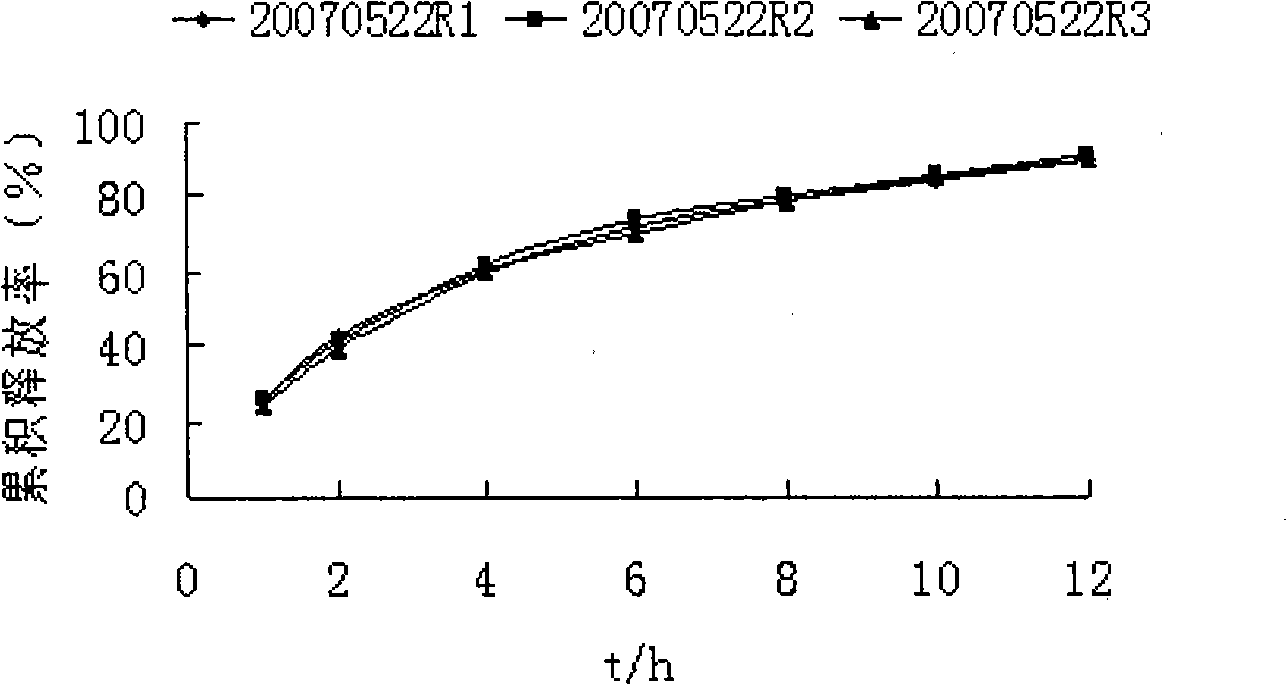
Image
Examples
Embodiment 1
[0033] Preparation of Metformin Hydrochloride Sustained Release Pellets
[0034] The prescription is (per 1000 capsules):
[0035] Metformin Hydrochloride 500g
[0036] Sucrose or lactose 200g
[0037] Microcrystalline Cellulose 300g
[0038] Surelease Water Dispersion (Colorcon) 1000g
[0039] Distilled water 1000g
[0040] Metformin hydrochloride, sucrose or lactose, and microcrystalline cellulose were pulverized respectively, passed through a 100-mesh sieve, mixed evenly, and distilled water was used as a binder, with an amount of 330ml, and the metformin hydrochloride drug-containing pellets were prepared by extrusion spheronization. Among them, the extrusion rate is 30rpm, the spheronization speed is 2500rpm, the spheronization time is 2min, the finished product is dried at 50°C, and the pellets between 700 and 900μm are sieved, which are drug-containing pellets. Add 1000g of distilled water to 1000g of Surelease aqueous dispersion, stir and mix evenly, as a coating ...
Embodiment 2
[0051] Preparation of metformin hydrochloride sustained-release pellets:
[0052] The prescription is (per 1000 capsules):
[0053] Metformin Hydrochloride 500g
[0054] Sucrose or lactose 150g
[0055] Microcrystalline Cellulose 250g
[0056] Surelease Water Dispersion 792g
[0057] Distilled water 792g
[0058] Grind metformin hydrochloride, sucrose or lactose, and microcrystalline cellulose respectively, pass through a 100-mesh sieve, mix well, use distilled water as a binder, and use 275 ml. Metformin hydrochloride drug-containing pellets were prepared by extrusion-spheronization method, wherein the extrusion rate was 35rpm, the spheronization speed was 2500rpm, the spheronization time was 2min, the finished product was dried at 50°C, and the pellets between 700 and 900μm were sieved to obtain the drug-containing pellets . Add 792g of distilled water to 792g of Surelease aqueous dispersion, stir, mix evenly, and use it as a coating solution. Take the drug-containing...
Embodiment 3
[0069] Preparation of metformin hydrochloride sustained-release pellets:
[0070] The prescription is (per 1000 capsules):
[0071] Metformin Hydrochloride 500g
[0072] Sucrose or lactose 100g
[0073] Microcrystalline Cellulose 150g
[0074] Surelease water dispersion 600g
[0075] Distilled water 600g
[0076] Metformin hydrochloride, sucrose or lactose, and microcrystalline cellulose were respectively crushed through a 100-mesh sieve, mixed evenly, and distilled water was used as a binder, and the dosage was 150ml. Metformin hydrochloride drug-containing pellets were prepared by extrusion-spheronization method. The extrusion rate is 25rpm, the spheronization speed is 2200rpm, and the spheronization time is 5min. The finished product is dried at 40°C, and the pellets between 700 and 900μm are sieved, which are drug-containing pellets. Add 600g of distilled water to 600g of Surelease aqueous dispersion, stir, and mix evenly, as a coating solution. The drug-containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com