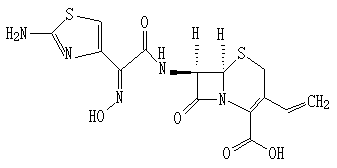
Cefdinir granule and preparation method thereof
A technology of cefdinir granules and granules, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and bulk delivery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
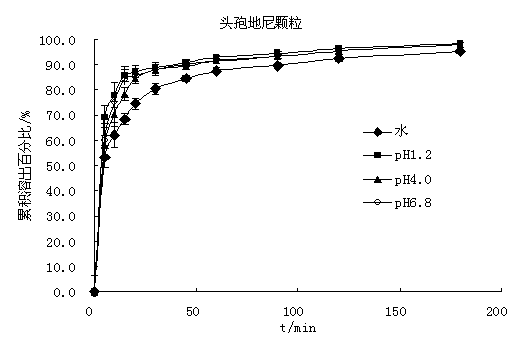
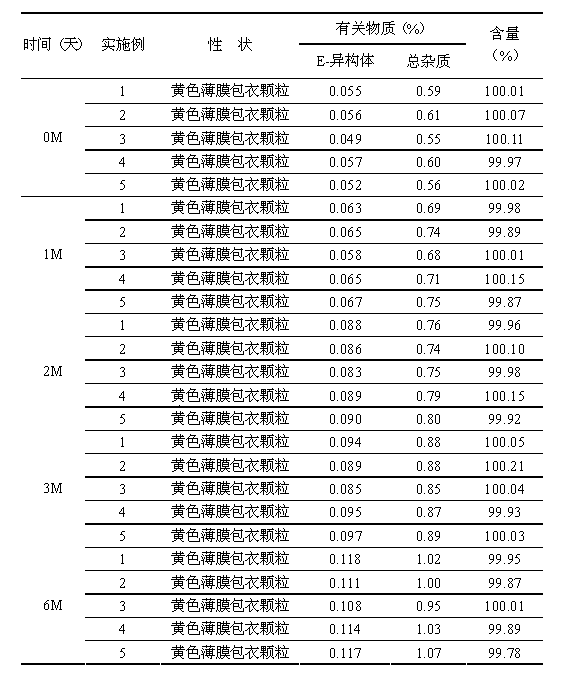
Image
Examples
Embodiment 1
[0048] Cefdinir 100g
[0049] Lactose 600g
[0050] Sucrose 600g
[0051] Low-substituted hydroxypropyl cellulose 250g
[0052] Orange flavor 10g
[0054] Micronized silica gel 5g
[0055] Hydroxypropyl Cellulose Q.S.
[0056] Talc Q.S.
[0057]
[0058] Made into 1000 pieces
[0059] Preparation:
[0060] The excipients are crushed through 100 sieves, and set aside; the active ingredient is micronized so that the particle size is less than 10 μm, set aside. The active ingredient cefdinir and the prescribed amount of lactose, sucrose, low-substituted hydroxypropyl cellulose, orange essence, saccharin sodium, and micropowder silica gel are added in equal amounts, and mixed evenly through a 100-mesh sieve to obtain a mixed powder, hydroxypropyl cellulose The soft material is made of cellulose-based 70% alcohol solution, and the pellets are prepared by extrusion and spheronization technology. The mesh size of the pellets is selected to be a...
Embodiment 2
[0062] Cefdinir 100g
[0063] Lactose 600g
[0064] Sucrose 600g
[0065] Low-substituted hydroxypropyl cellulose 250g
[0066] Orange flavor 10g
[0068] Micronized silica gel 5g
[0069] Hydroxypropyl Cellulose Q.S.
[0070] Talc Q.S.
[0071]
[0072] Made into 1000 pieces
[0073] Preparation:
[0074] The excipients are crushed through 100 sieves, and set aside; the active ingredient is micronized so that the particle size is less than 10 μm, set aside. The active ingredient cefdinir and the prescribed amount of lactose, sucrose, low-substituted hydroxypropyl cellulose, orange essence, sodium saccharin, and micropowder silica gel are added in equal amounts, and mixed evenly through a 100-mesh sieve to obtain a mixed powder, which is centrifuged Prepare granules by granulation, select the granules with a mesh size of about 40 mesh, coat the granules with a colored film, and pack them in one-piece tapes to obtain the product.
Embodiment 3
[0076] Cefdinir 100g
[0077] Lactose 1200g
[0078] Croscarmellose Sodium 150g
[0079] Tartaric acid 50g
[0080] Sodium bicarbonate 50g
[0081] Cherry essence 10g
[0082] Sodium saccharin 10g
[0083] Micronized silica gel 5g
[0084] Hydroxypropyl Cellulose Q.S.
[0085] Talc Q.S.
[0086]
[0087] Made into 1000 pieces
[0088] Preparation:
[0089] The excipients are crushed through 100 sieves, and set aside; the active ingredient is micronized so that the particle size is less than 10 μm, set aside. The active ingredient cefdinir and the prescribed amount of lactose, croscarmellose sodium, tartaric acid, sodium bicarbonate, cherry essence, sodium saccharin, and micropowder silica gel are added in equal amounts and mixed evenly through a 100-mesh sieve to obtain Mixed powder, soft material made of 70% hydroxypropyl cellulose alcohol solution, granules are prepared by extrusion and spheronization technology, the mesh size of the granules is about 40 mesh, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com