Omeprazole enteric capsule and preparation method thereof
A technology of omeprazole enteric and omeprazole is applied in the field of omeprazole enteric-coated capsules, the preparation of omeprazole enteric-coated capsules, and the field of omeprazole enteric-coated pellets, which can solve difficult realization, etc. problems, to achieve consistent peak time, improve in vitro dissolution, and achieve the effect of good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 The processing of omeprazole micronization
[0062] Weigh 50Kg of omeprazole raw material passing through a 80 mesh sieve, put it into an MQL03 jet mill (manufactured by Weifang Erpai Powder Technology Equipment Co., Ltd.) for micronization, and pass through a 325 mesh sieve to obtain micronized Ogilvy Prazole 46.8Kg, yield is 93.6%. Take the micronized omeprazole sample and detect it with a Mastersizer 2000 Malvern laser particle size analyzer (manufactured by Malvern Instruments, UK). The D50 is 2.93um and the D90 is 7.32um.
Embodiment 2
[0063] The preparation of embodiment 2 omeprazole enteric-coated capsules
[0064] (1) Preparation of omeprazole drug-loaded layer
[0065] Weigh 1180 g of pulverized mannitol and set aside. Under the state of stirring, 20 g of disodium hydrogen phosphate weighed was added to 391 g of purified water. After fully stirring until clear and transparent, 2 g of sodium lauryl sulfate (and 1 g of Tween 80) were added. After stirring until uniform and no lumps, add 200g of omeprazole, stir for not less than 15 minutes, and set aside. Weigh mannitol, lactose, microcrystalline cellulose, high-substituted hydroxypropyl cellulose (and low-substituted hydroxypropyl cellulose) according to the prescription amount, put them into the wet mixing granulator in turn, set reasonable process parameters, and wait for the preparation grain. Weigh the granulation solution, set reasonable process parameters, and granulate. Put the wet granules into the extrusion spheronizer, according to the reas...
Embodiment 3
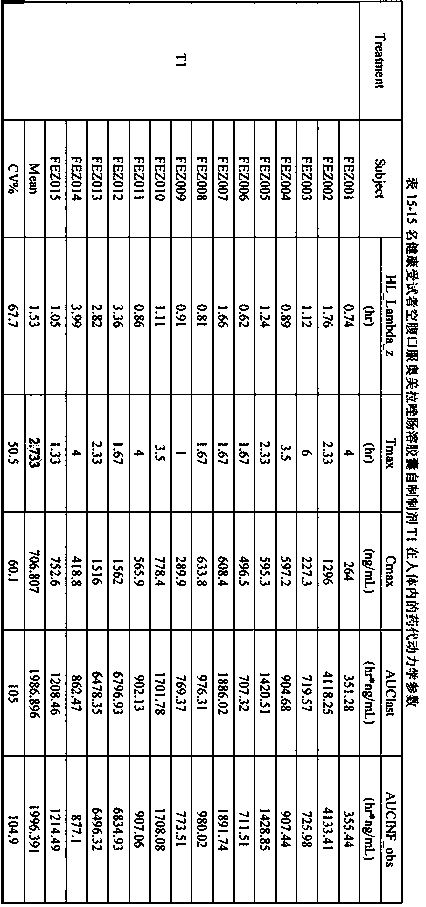
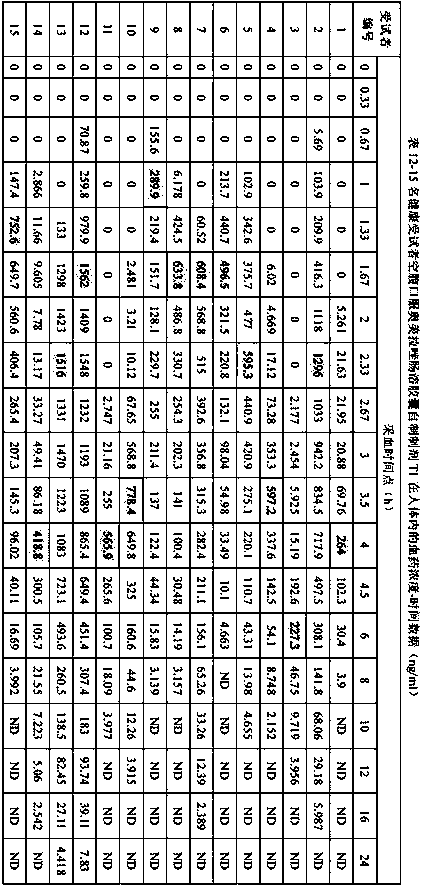
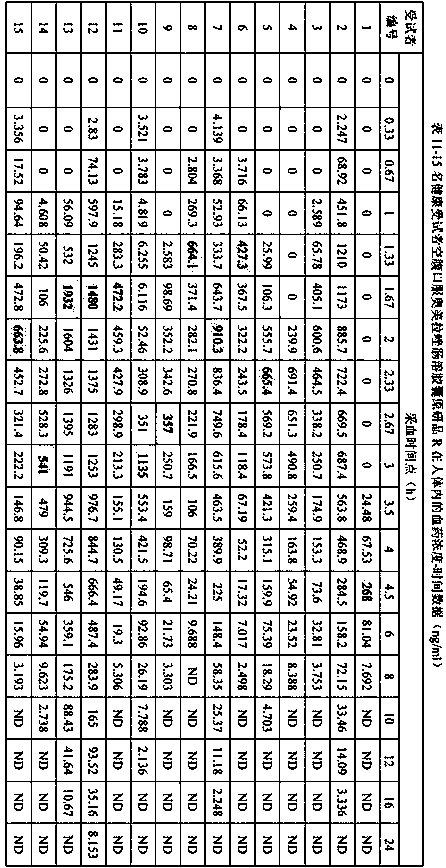
[0074] Example 3 Comparison of the in vitro dissolution characteristics of omeprazole enteric-coated capsules (T1, T2) prepared by the preparation method of the present invention and the original research product of omeprazole enteric-coated capsules (R)
[0075] Refer to the "Chinese Pharmacopoeia" 2015 edition two monographs on the acid resistance and dissolution determination method of omeprazole enteric-coated capsules, acid resistance, water, pH6.8 phosphate buffer using high performance liquid chromatography, chromatographic conditions according to Austrian Under the item of content determination of meprazole enteric-coated capsules; since omeprazole enteric-coated capsules have been degraded in the process of dissolution in pH6.0 phosphate buffer solution, the determination by high performance liquid chromatography is not applicable, so Determination by UV spectrophotometry.
[0076] High performance liquid chromatography chromatographic conditions: use octylsilane bonded...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com