Compound with metformin and repaglinide, preparation method thereof and application thereof
A technology of metformin hydrochloride and compound recipe, applied in the field of medicine, can solve the problems such as no literature report of metformin hydrochloride, and achieve the effects of reducing the number of times of taking, simplifying use, and improving the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
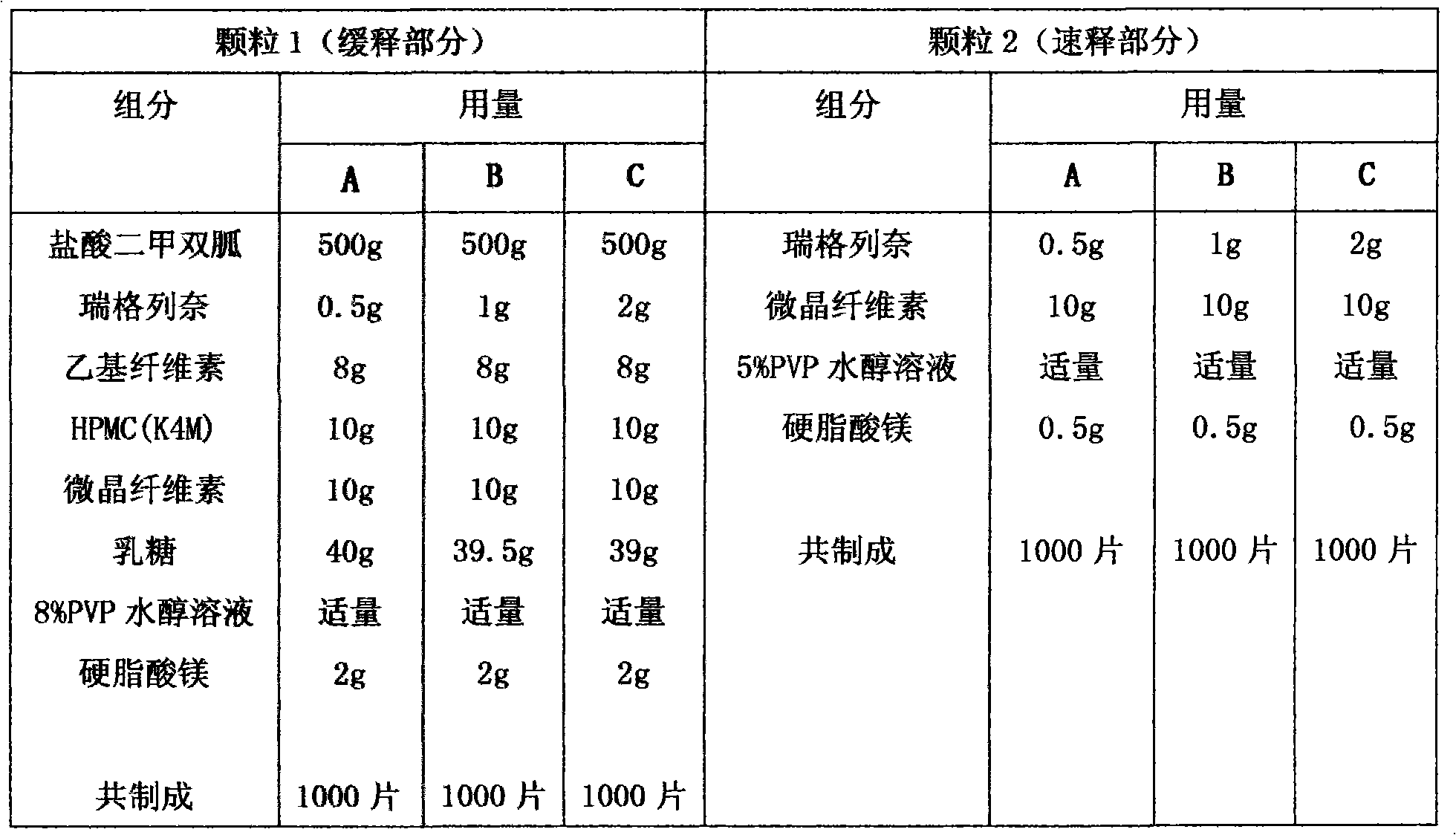
[0031] Embodiment 1 sustained-release tablet, sustained-release granule, sustained-release capsule
[0032] prescription
[0033]
[0034] Preparation:
[0035] Particle 1: Pass the main ingredient and auxiliary materials through 80-mesh sieve for later use. Take metformin hydrochloride, repaglinide, ethyl cellulose, HPMC (K4M), microcrystalline cellulose, lactose according to the prescription, after mixing with the method of equal increments, add 8% PVP hydroalcoholic solution, make the material reach " Knead into agglomerates, touch it and it will be made into a soft material. The soft material is granulated through a 24-mesh sieve. After drying at 60°C-65°C, it is granulated through a 18-mesh sieve. uniform.
[0036] Particle 2: Pass the main ingredient and auxiliary materials through 80-mesh sieve for later use. Weigh repaglinide, HPMC (K4M), microcrystalline cellulose, and lactose according to the prescription, and mix them evenly with the method of equal increment...
Embodiment 2
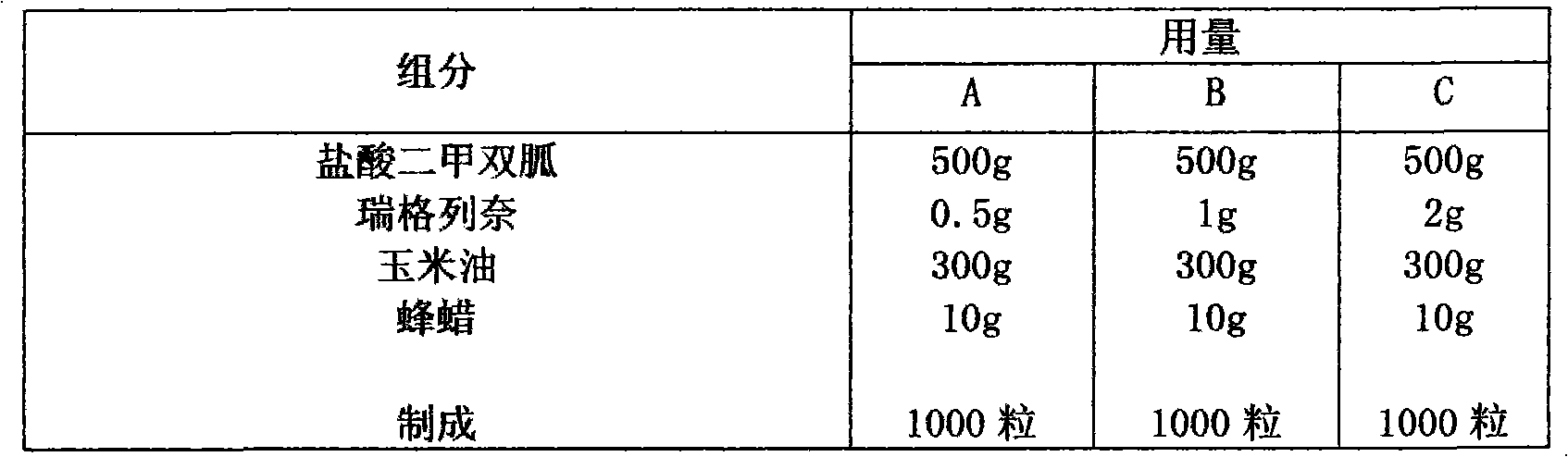
[0040] Embodiment 2 liquid capsule
[0041] prescription
[0042]
[0043] Preparation:
[0044] Pass the metformin hydrochloride and repaglinide through a 120-mesh sieve and set aside. Weigh beeswax according to the prescription, add it to corn oil, heat and stir until dissolved, add the prescribed amount of metformin hydrochloride and repaglinide, mix and stir thoroughly to make a suspension, put it into a suitable transparent hard capsule, heat seal, and obtain .
Embodiment 4
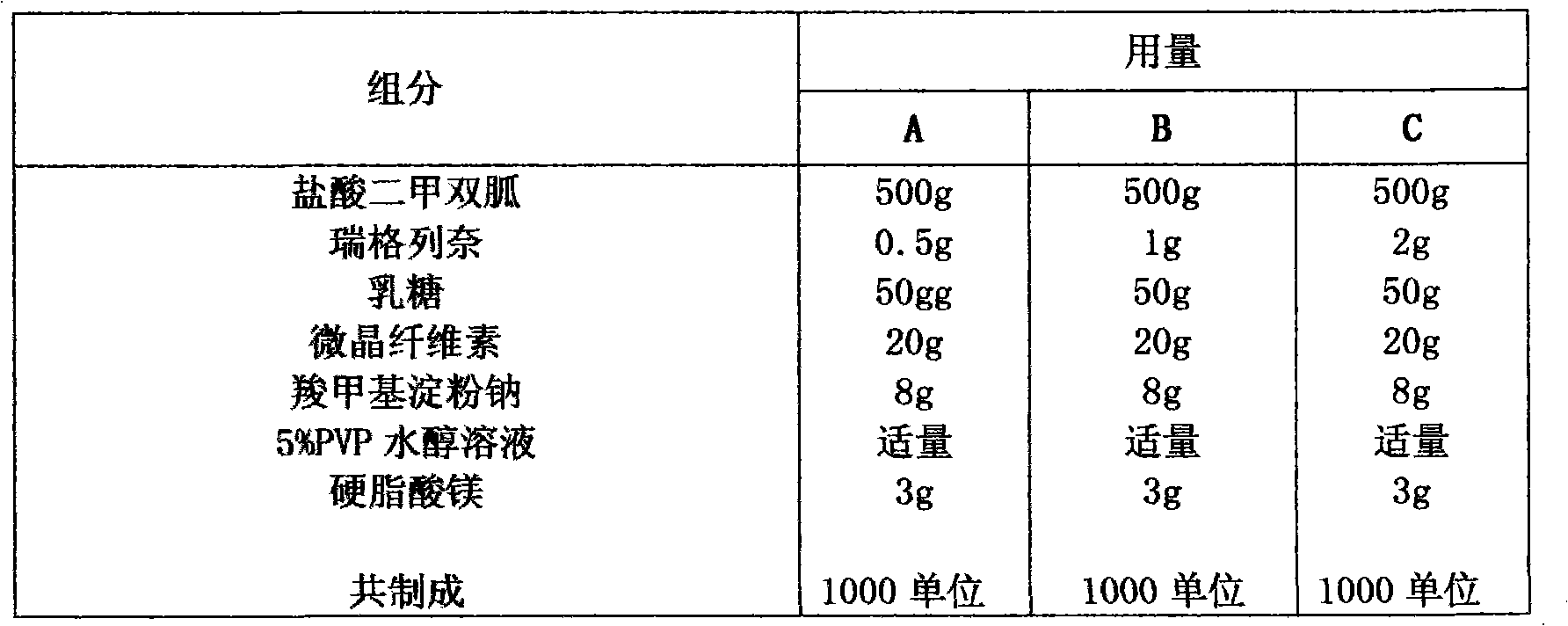
[0045] Embodiment 4 Granules, tablets, capsules
[0046] prescription
[0047]
[0048] Preparation:
[0049] Pass the main ingredient and auxiliary materials through a 100-mesh sieve for later use. Weigh metformin hydrochloride, repaglinide and other excipients except magnesium stearate according to the prescription, mix them in equal increments, add binder to make soft material, and pass the soft material through a 24-mesh sieve to granulate, at 60°C After left and right drying, pass through a 18-mesh sieve for granulation, then add magnesium stearate and mix well.
[0050] The above granules can be directly divided into granules.
[0051] The above-mentioned granules are selected and packed in suitable capsule shells to make capsules.
[0052] The above granules are compressed with a suitable die to form tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com