Slowly released clamycin capsule
A technology for capsules and sustained-release drugs, which is applied in the field of clarithromycin sustained-release capsules and its preparation, and can solve problems such as no clarithromycin sustained-release capsules and no products on the market.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
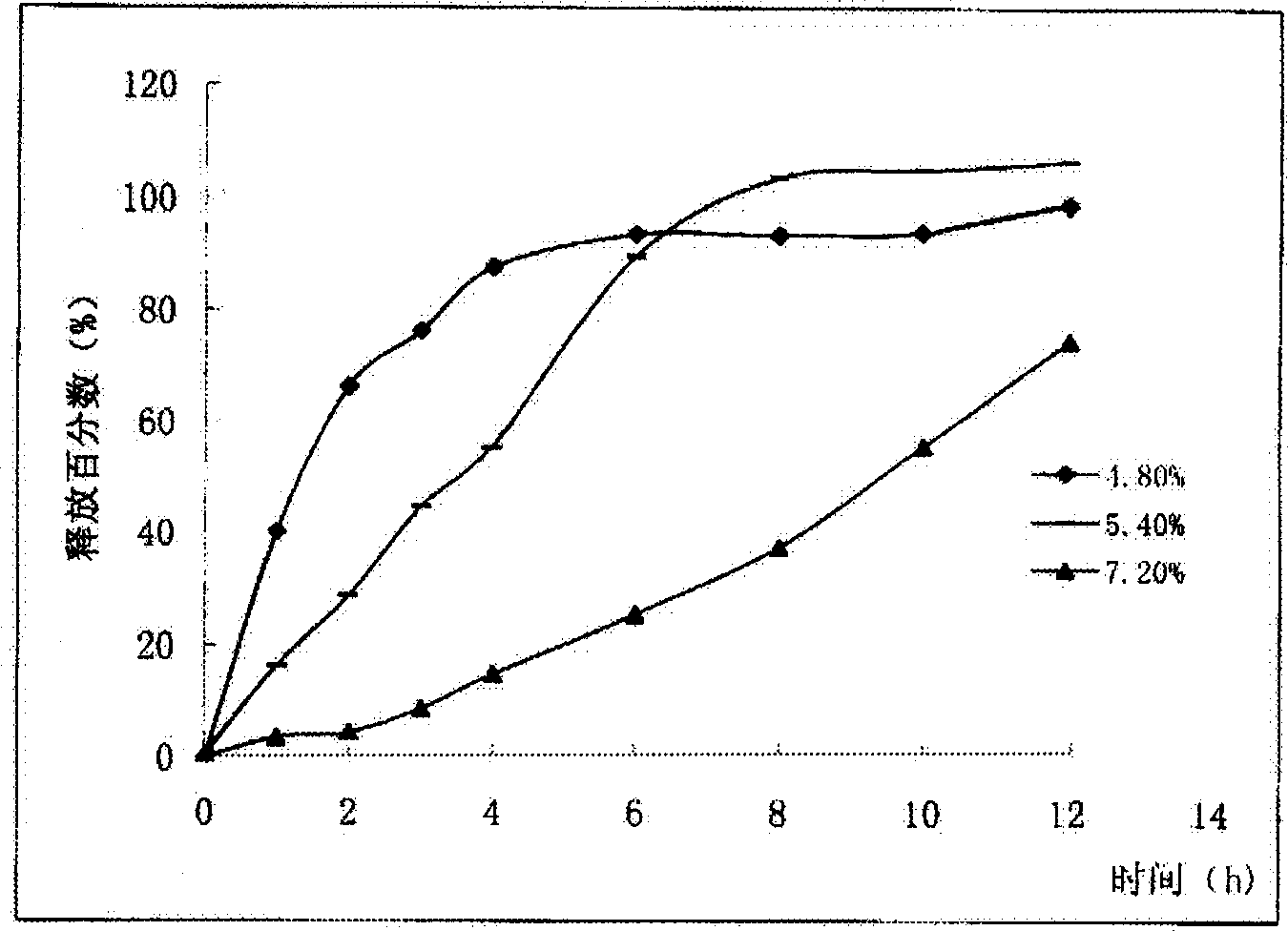
Image
Examples
Embodiment 1
[0017] (1) Preparation of mother core (blank pellet core):
[0018] Preparation: Commercially available blank pellets can be used, or a centrifugal coating granulator, high-speed stirring granulator, extrusion spheronizer or fluidized bed granulator can be used to prepare.
[0019] (2) Prescription of drug-containing pill core:
[0020] Clarithromycin 250g
[0021] Microcrystalline Cellulose 70g
[0022] 40g powdered sugar
[0023] 5% hypromellose appropriate amount
[0024] Sodium Lauryl Sulfate 0.3g
[0025] Make 1000 capsules
[0026] Preparation: Weigh 400g of the 40-mesh mother core, put it in the material chamber of a centrifugal coating granulator or a fluidized bed coating granulator, and mix clarithromycin powder, sugar powder, microcrystalline cellulose and ten Sodium dialkylsulfate mixed powder (120 mesh), put solid feeding chamber, 5% hydroxypropyl methylcellulose aqueous solution is binder, start centrifugal coating granulator o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com