Statins zinc salt-containing blood fat-reducing composite
A composition and a blood lipid-lowering technology, applied in the field of medicine, can solve the problems of high production cost, poor stability, complicated calcium salt production process, etc., and achieve the effect of significant curative effect and cholesterol reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
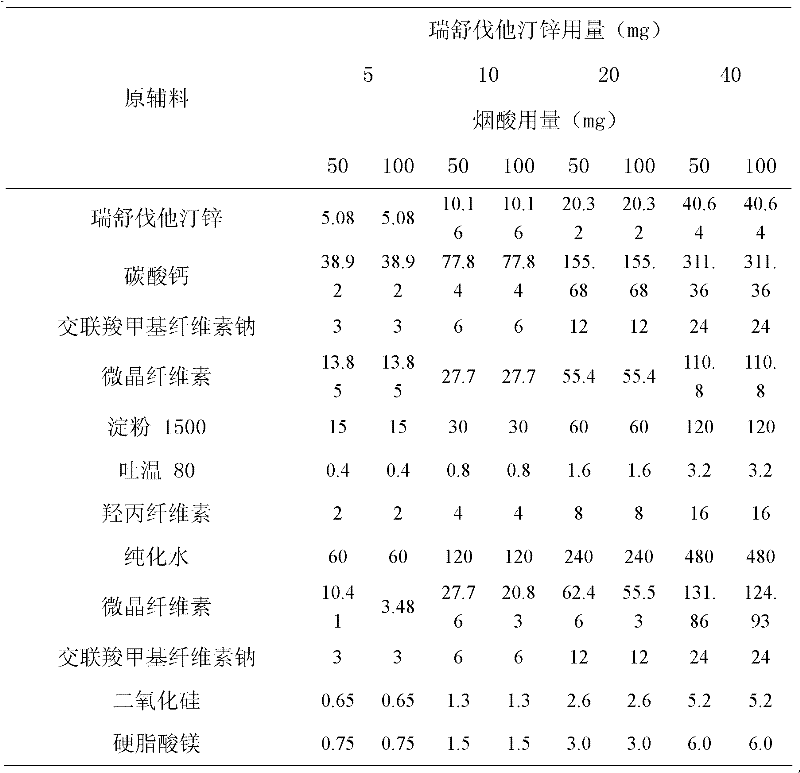
[0017] Example 1 Compound Rosuvastatin Zinc Nicotinic Acid Tablets
[0018]
[0019] Preparation:
[0020] 1. Add the prescribed amount of Tween 80 to purified water at 50°C, stir well to dissolve, add hydroxypropyl cellulose to hydrate it, and form a binder.
[0021] 2. The prescribed amount of rosuvastatin zinc, calcium carbonate, microcrystalline cellulose, starch 1500, and croscarmellose sodium are fully mixed uniformly by equal amount addition method.
[0022] 3. Add the binder soft material obtained in step 1 to the mixed powder obtained in step 2, and granulate with an 18-mesh sieve.
[0023] 4. Dry the granules obtained in step 3, and control the moisture content to be no more than 2.0%.
[0024] 5. Add nicotinic acid, microcrystalline cellulose, croscarmellose sodium, and silicon dioxide to the granules obtained in step 4 and mix well. Pass through a 24-mesh sieve.
[0025] 6. Add magnesium stearate to the mixed granules obtained in step 5, mix well, and press ...
Embodiment 2
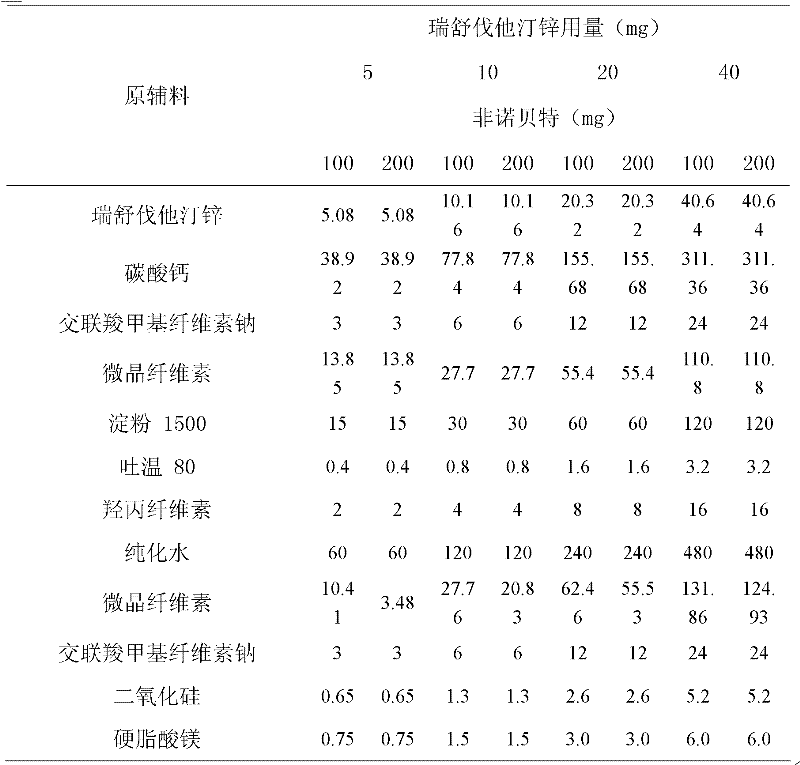
[0026] Example 2 Compound Rosuvastatin Zinc Fenofibrate Tablets
[0027]
[0028] Preparation:
[0029] 1. Add the prescribed amount of Tween 80 to purified water at 50°C, stir well to dissolve, add hydroxypropyl cellulose to hydrate it, and form a binder.
[0030] 2. The prescribed amount of rosuvastatin zinc, calcium carbonate, microcrystalline cellulose, starch 1500, and croscarmellose sodium are fully mixed uniformly by equal amount addition method.
[0031] 3. Add the binder soft material obtained in step 1 to the mixed powder obtained in step 2, and granulate with an 18-mesh sieve.
[0032] 4. Dry the granules obtained in step 3, and control the moisture content to be no more than 2.0%.
[0033] 5. Add fenofibrate, microcrystalline cellulose, croscarmellose sodium, and silicon dioxide to the granules obtained in step 4 and mix well. Pass through a 24-mesh sieve.
[0034] 6. Add magnesium stearate to the mixed granules obtained in step 5, mix well, and press into t...
Embodiment 3
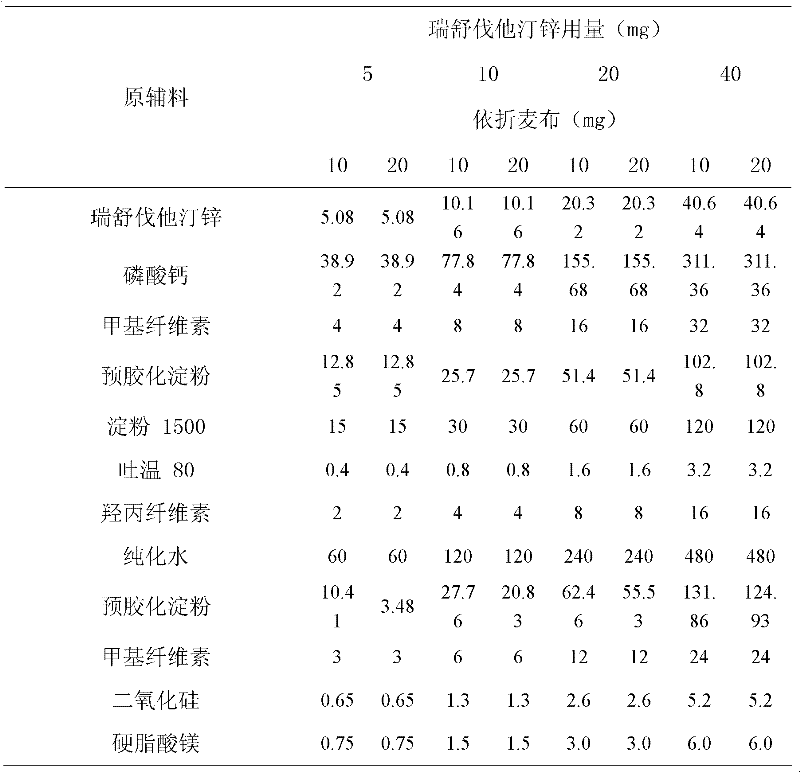
[0035] Example 3 Compound Rosuvastatin Ezetimibe Tablets
[0036]
[0037] Preparation:
[0038] 1. Add the prescribed amount of Tween 80 to purified water at 50°C, stir well to dissolve, add hydroxypropyl cellulose to hydrate it, and form a binder.
[0039] 2. Fully mix the prescription amount of rosuvastatin zinc, calcium phosphate, pregelatinized starch, starch 1500, and methyl cellulose with the method of equal volume addition.
[0040] 3. Add the binder soft material obtained in step 1 to the mixed powder obtained in step 2, and granulate with an 18-mesh sieve.
[0041] 4. Dry the granules obtained in step 3, and control the moisture content to be no more than 2.0%.
[0042] 5. Add ezetimibe, pregelatinized starch, methylcellulose and silicon dioxide to the granules obtained in step 4 and mix well. Pass through a 24-mesh sieve.
[0043] 6. Add magnesium stearate to the mixed granules obtained in step 5, mix well, and press into tablets to obtain final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com