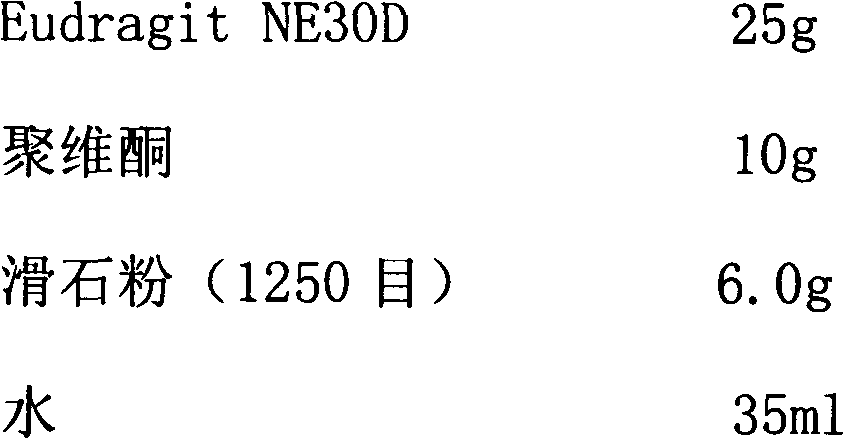Metformin hydrochloride sustained-release capsule and its preparation method
A technology of metformin hydrochloride and metformin hydrochloride, applied in the field of medicine, can solve the problems of poor penetrability of the lower small intestine and large intestine, easy to cause adverse reactions, and large fluctuations in blood drug concentration, so as to avoid adverse reactions, facilitate swallowing, The effect of avoiding pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
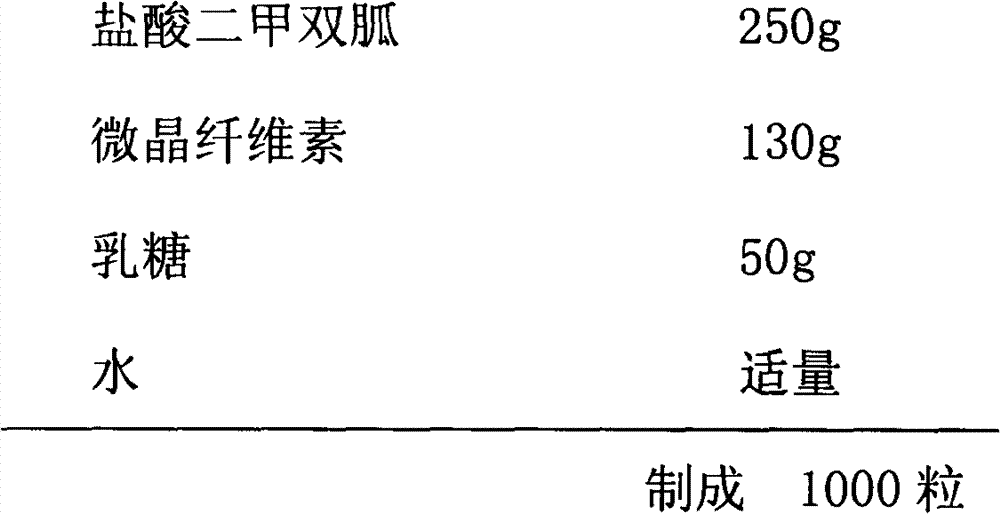
[0020] Cystic Heart Prescription:
[0021]
[0022] Coating prescription:
[0023] Eudragit NE30D 35g
[0024] Talc powder (1250 mesh) 6.3g
[0025] water 35ml
[0026] Concrete preparation process comprises the following steps:
[0027] (1) Preparation of micropills: preparation of drug-containing pellet cores by extrusion spheronization
[0028] In the present invention, after fully mixing the active ingredients and the diluent, the soft material is prepared with water. Select a 0.8mm sieve plate, operate at an extrusion frequency of 30rpm, a spheronization frequency of 50rpm, and a spheronization time of 5min, sieve the prepared pellets, and select 24-32 mesh particles.
[0029] (2) Sustained-release coating: coating by fluidized bed coating method
[0030] The film-forming material acrylic resin polymer material (Eudragit NE30D), talcum powder and water are uniformly mixed as a coating solution. Get the pellets equivalent to 250mg of metformin hydrochloride, put ...
Embodiment 2
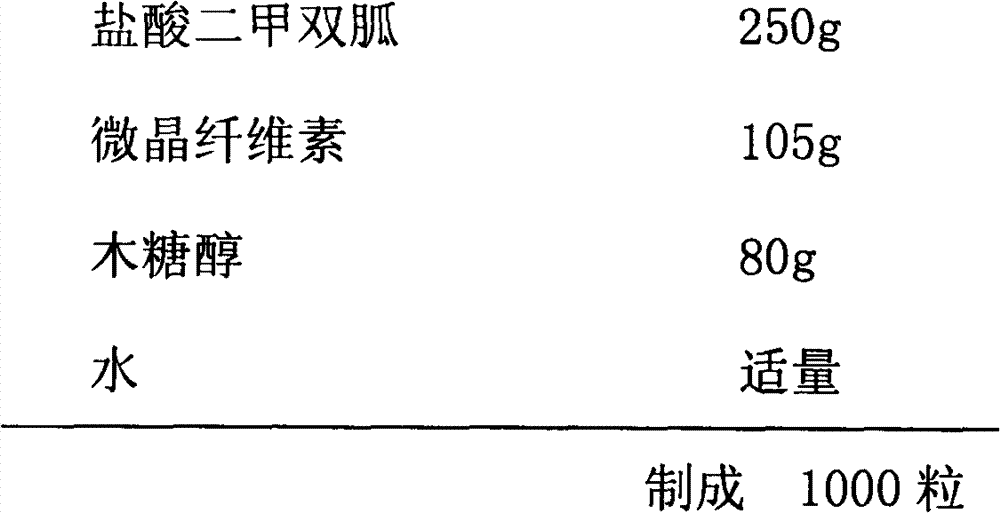
[0034] Cystic Heart Prescription:
[0035]
[0036] Coating prescription:
[0037]
[0038] Concrete preparation process comprises the following steps:
[0039] (1) Preparation of micropills: preparation of drug-containing pellet cores by extrusion spheronization
[0040] In the present invention, after fully mixing the active ingredients and the diluent, the soft material is prepared with water. Select a 0.8mm sieve plate, operate at an extrusion frequency of 35rpm, a spheronization frequency of 55rpm, and a spheronization time of 5min, sieve the prepared pellets, and select 24-32 mesh particles.
[0041] (2) Sustained-release coating: coating by fluidized bed coating method
[0042] The film-forming material acrylic resin polymer material (Eudragit NE30D), talcum powder and water are uniformly mixed as a coating solution. Get the micropills equivalent to metformin hydrochloride 250mg, drop in the fluidized bed coating machine, with air volume 14L / min, spray speed 2...
Embodiment 3
[0046] Cystic Heart Prescription:
[0047]
[0048] Coating prescription:
[0049]
[0050] Concrete preparation process comprises the following steps:
[0051] (1) Preparation of micropills: preparation of drug-containing pellet cores by extrusion spheronization
[0052]In the present invention, after fully mixing the active ingredients and the diluent, the soft material is prepared with water. Select a 0.8mm sieve plate, operate at an extrusion frequency of 30rpm, a spheronization frequency of 50rpm, and a spheronization time of 5min, sieve the prepared pellets, and select 24-32 mesh particles.
[0053] (2) Sustained-release coating: coating by fluidized bed coating method
[0054] The film-forming material acrylic resin polymer material (Eudragit NE30D), talcum powder and water are uniformly mixed as a coating solution. Get the micropills equivalent to metformin hydrochloride 250mg, drop in the fluidized bed coating machine, with air volume 13L / min, spray speed 28...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com