Core-shell type pre-charged chemotherapy medicine embolization microsphere and preparation method thereof
A chemotherapy drug and embolization microsphere technology, applied in the field of biomedicine, can solve the problems of reducing local efficacy, increasing adverse reactions, high drug loss rate, etc., to achieve the effect of changing distribution and kinetics, improving bioavailability, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Core-shell prepacked paclitaxel embolization microspheres and their preparation
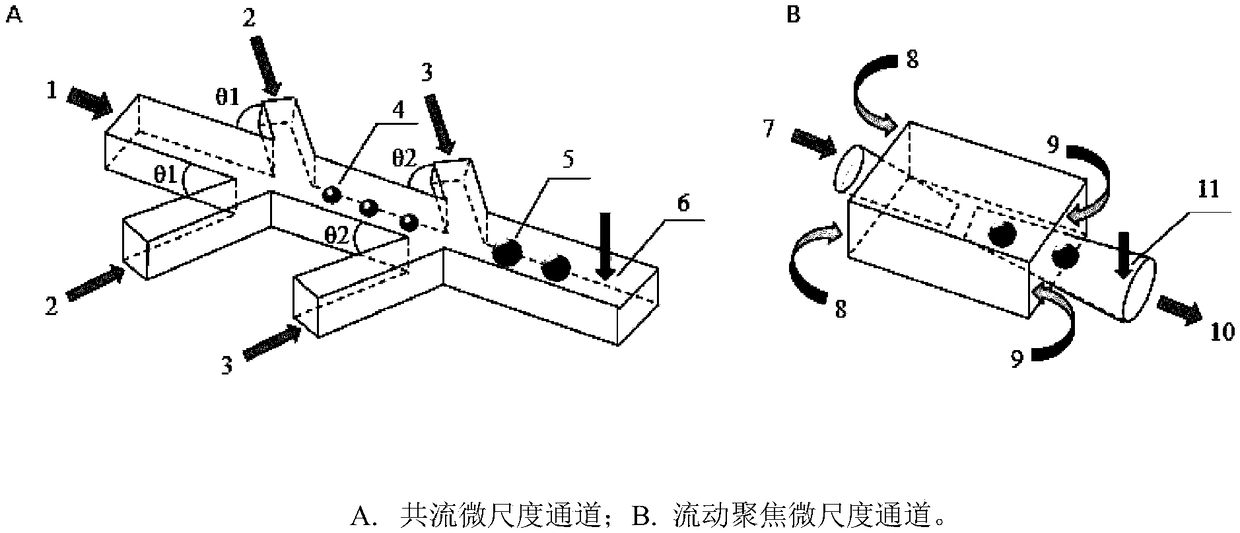
[0069] select figure 1 (A) co-flow micro-scale channel, preparing O / W / O double emulsion micro-droplets, wherein, θ1=90°, θ2=90°; the diameter of the inner oil phase fluid channel 1 in the micro-scale channel is 200 microns, and the middle The diameter of the water phase fluid channel 2 is 200 microns, the diameter of the collection micro channel 4 is 250 microns; the diameter of the outer oil phase fluid channel 3 is 250 microns, and the diameter of the collection micro channel 5 is 400 microns.
[0070]The inner oil phase is a dichloromethane solution containing 8% PLGA, in which 400 μg / mL paclitaxel is dissolved; the middle water phase is a mixed aqueous solution of 1.5% sodium alginate and 2% polyvinyl alcohol, and the outer oil phase is a solution containing 2% EM90. Liquid paraffin oil. The inner oil phase, the middle water phase and the outer oil phase were pumped into c...
Embodiment 2
[0072] Example 2: Core-shell prepacked ginsenoside embolization microspheres and their preparation
[0073] The micro-scale channel is as described in Example 1 to prepare O / W / O double emulsion micro-droplets. The difference is that the diameter of the inner oil phase fluid channel 1 in the microscale channel is 150 microns, the diameter of the middle water phase fluid channel 2 is 150 microns, the diameter of the collection micro channel 4 is 200 microns; the diameter of the outer oil phase fluid channel 3 is 200 microns, The collection microchannel 5 has a diameter of 300 microns.
[0074] The inner oil phase is selected from dimethyl carbonate solution containing 6% PLGA, in which 30 μg / ml ginsenoside is dissolved; Polyvinyl alcohol mixed aqueous solution, the outer oil phase is selected from soybean oil containing 2% DC749. The inner oil phase, middle water phase and outer oil phase were pumped into channels 1, 2, and 3 at constant flow rates of 0.7 mL / h, 3.0 mL / h, and 6...
Embodiment 3
[0076] Example 3: Core-shell prepacked doxorubicin embolization microspheres and their preparation
[0077] select figure 1 (B) Micro-scale channel of flow-focusing structure to prepare W / O / W double-emulsion droplets. The outlet diameter of the aqueous phase fluid channel 7 in the micro-scale channel is 50 microns, and the inlet diameter of the collection micro channel 10 is 120 microns.
[0078] The inner water phase is a mixed aqueous solution of 20% PEG and 2% sodium alginate, and 200 μg / mL of doxorubicin is dissolved in it; the middle oil phase is made of dodecane containing 2% EM90, and the outer water phase is 4% polyvinyl alcohol aqueous solution . The inner water phase, the middle oil phase and the outer water phase were pumped into channels 7, 8, and 9 at constant flow rates of 0.8 mL / h, 2.0 mL / h, and 13.0 mL / h, respectively. Double emulsion micro-droplets are generated at the intersection of channels 7 and 10.
[0079] The droplets were collected and added to a 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com