Crystalline form I of acotiamide hydrochloride hydrate, preparation method therefor and use thereof
A technology of acotiamide hydrochloride and acotiamide, applied in the field of chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of Acotinamide Hydrochloride Monohydrate
[0032] Step (1) Preparation of Acotamide Hydrochloride
[0033] Take 3.0 g of free acotiamide, add 30 ml of isopropanol, and heat to 50°C to dissolve. At this temperature, add 4g of 35% hydrochloric acid aqueous solution, stir for 5h, and cool to 15°C naturally. Filter and wash the filter cake with 20 ml isopropanol. It was dried at 50°C to obtain 2.6g crude acotiamine hydrochloride.
[0034] Step (2) Refining of Acotamide Hydrochloride Monohydrate
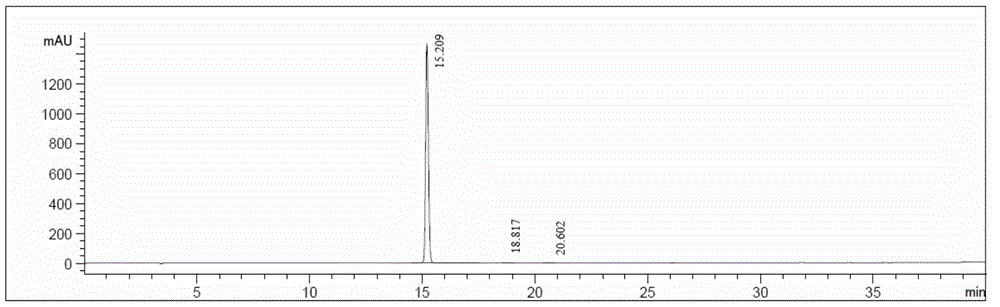
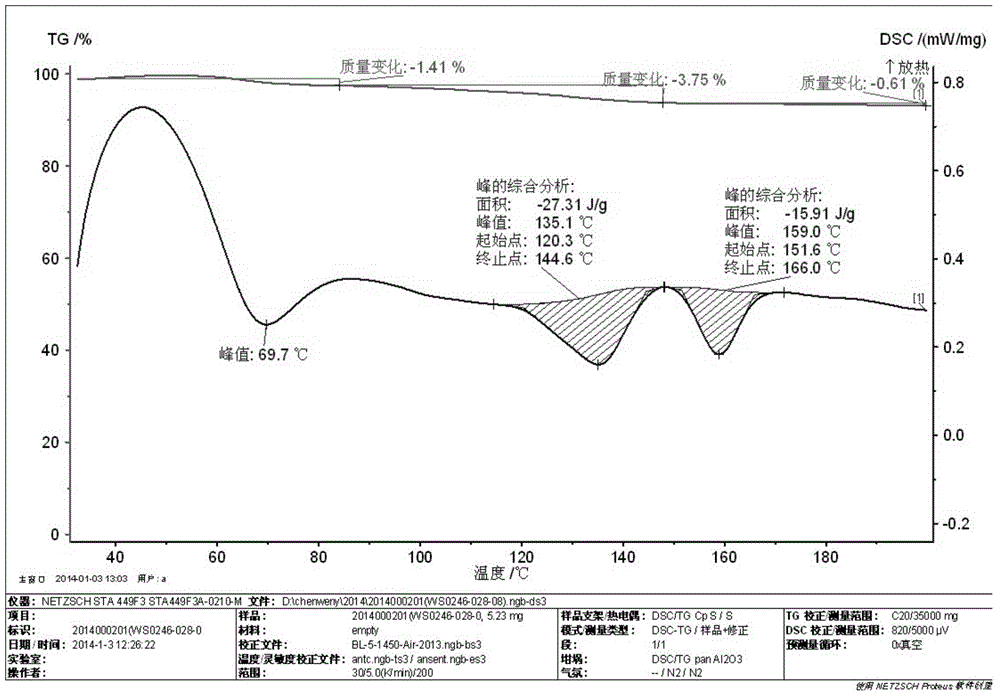
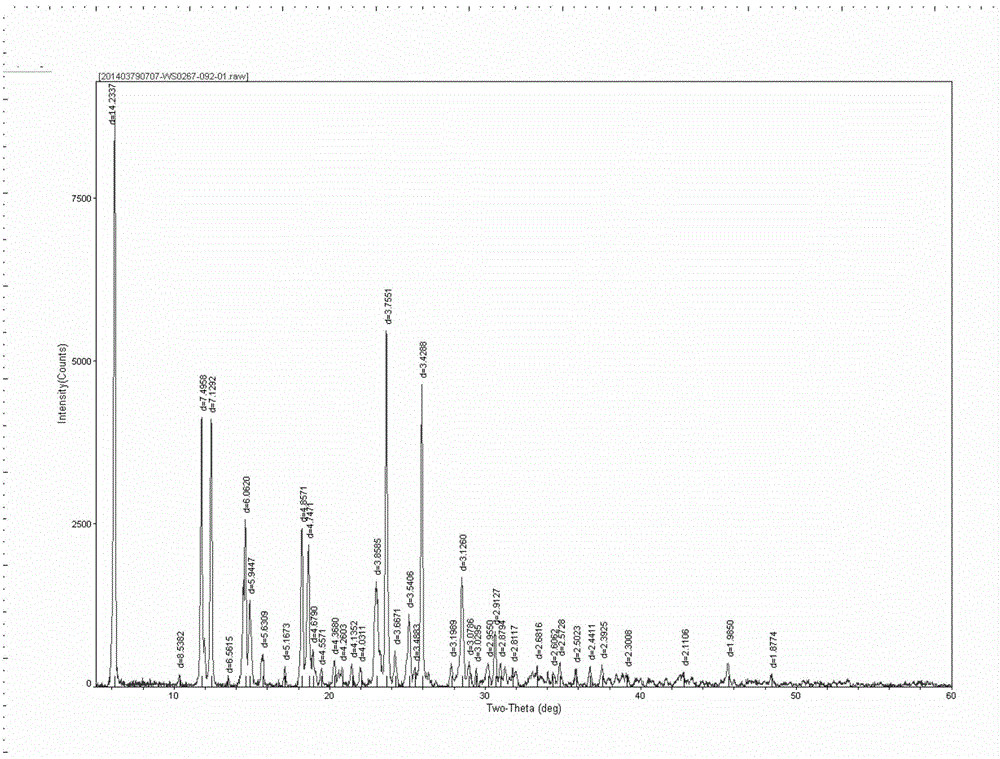
[0035] Take 2.6 g of the crude acotiamine hydrochloride prepared above, add 25 ml of a 70% isopropanol aqueous solution by volume, and heat to 70° C. to dissolve. Stir for 4h. Filter and wash the filter cake with 10 ml isopropanol. Then dry at 50°C for 6h. Finally, 2.36 g of acotiamide hydrochloric acid hydrate was obtained with a yield of 88.0%. The purity was 99.6% by HPLC, and the moisture value was 3.33% by the moisture analyzer. After thermal weight loss and...
Embodiment 2
[0037] Example 2 Synthesis of Acotinamide Hydrochloride Monohydrate
[0038] Step (1) Preparation of Acotamide Hydrochloride
[0039] Take 30g of free acotiamine, add 300ml of methanol, and heat to 70°C to dissolve. At this temperature, add 40g of 20% hydrochloric acid aqueous solution, stir for 5h, and cool to 15°C naturally. Filter and wash the filter cake with 50 ml methanol. Dry at 70℃. Finally, 29.2 g of crude acotiamine hydrochloride was obtained.
[0040] Step (2) Preparation of Acotamide Hydrochloride Hydrate
[0041] Take 29.2 g of the crude acotiamine hydrochloride prepared above, add 300 ml of an 80% isopropanol aqueous solution by volume, and heat to 70°C to dissolve. Stir for 4h. Filter and wash the filter cake with 100 ml isopropanol. Then dry at 60°C for 4h. Finally, 27.06 g of acotiamide hydrochloride hydrate was obtained, with a total yield of 89.7%. The purity was 99.5% by HPLC, and the moisture value was 3.33% by a moisture analyzer. After thermal weight los...
Embodiment 3
[0043] Example 3 Synthesis of Acotinamide Hydrochloride Monohydrate
[0044] Step (1) Preparation of Acotamide Hydrochloride
[0045] Take 130g of free acotiamine, add 450ml of methanol, and heat to 30°C to dissolve. At this temperature, 75g of 35% hydrochloric acid aqueous solution was added, stirred for 5h, and naturally cooled to 15°C. Filter and wash the filter cake with 100 ml methanol. Dry at 70℃. Finally, 110 g of crude acotiamine hydrochloride was obtained.
[0046] Step (2) Preparation of Acotamide Hydrochloride Hydrate
[0047] Take 110 g of the crude acotiamine hydrochloride prepared above, add 770 ml of an 80% isopropanol aqueous solution by volume, and heat to 70°C to dissolve. Stir for 4h. Filter and wash the filter cake with 100 ml isopropanol. Then dry at 60°C for 4h. Finally, 104.7 g of acotiamide hydrochloride hydrate was obtained with a yield of 92.1%. The purity was 99.7% by HPLC, and the moisture value was 3.33% by a moisture analyzer. After thermal weight...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com