Method for determining solvents residual in acotiamide hydrochloride bulk drug
A technology for acotiamide hydrochloride and residual solvents, applied in the field of determination of residual solvents in acotiamide hydrochloride raw materials, can solve problems that need to be improved, achieve rapid and efficient separation and content determination, high accuracy, and good quality assurance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
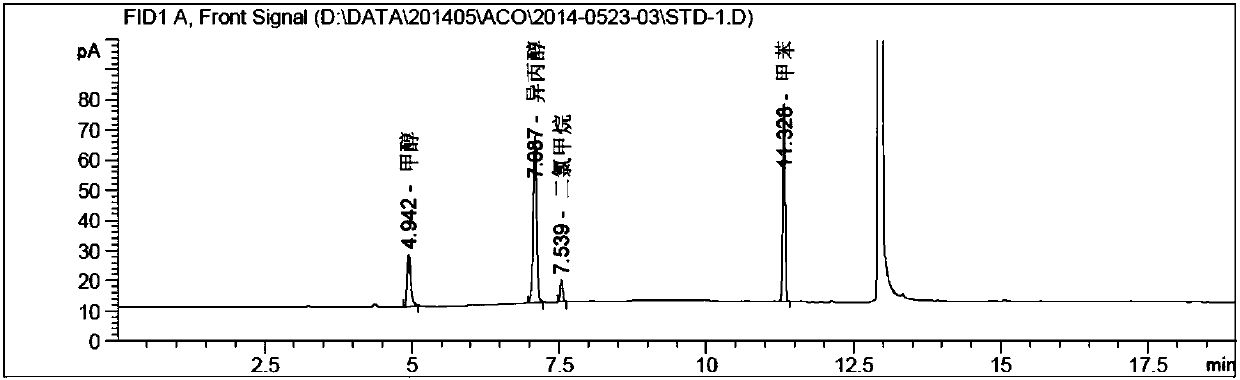
[0049] Instrument: Agilent 7890A-7697A headspace gas chromatograph, FID detector;
[0050]Chromatographic column: DB-624 (30m*0.53mm, 3.0μm);
[0051] Chromatographic parameters: carrier gas: nitrogen; carrier gas flow rate: 2.0ml / min; split ratio: 10:1;
[0052] Detector temperature: 250°C; Injection port temperature: 200°C;
[0053] Column temperature: the initial temperature is 40°C and kept for 5 minutes, then raised to 220°C at a rate of 20°C / min, and kept for 5 minutes;
[0054] Headspace detection parameters: heating box temperature: 80°C; quantitative loop temperature: 100°C; transfer line temperature: 110°C;
[0055] Headspace equilibration time: 20min; Cycle time: 30min; Injection time: 1.0min;
[0056] Pressure balance time: 0.2min.
[0057] 1. Methodological specificity
[0058] Blank solution: dimethylsulfoxide (DMSO).
[0059] Methanol stock solution: take about 0.30g of methanol, weigh it accurately, put it in a 50ml measuring bottle, dilute and dissolve i...
Embodiment 2
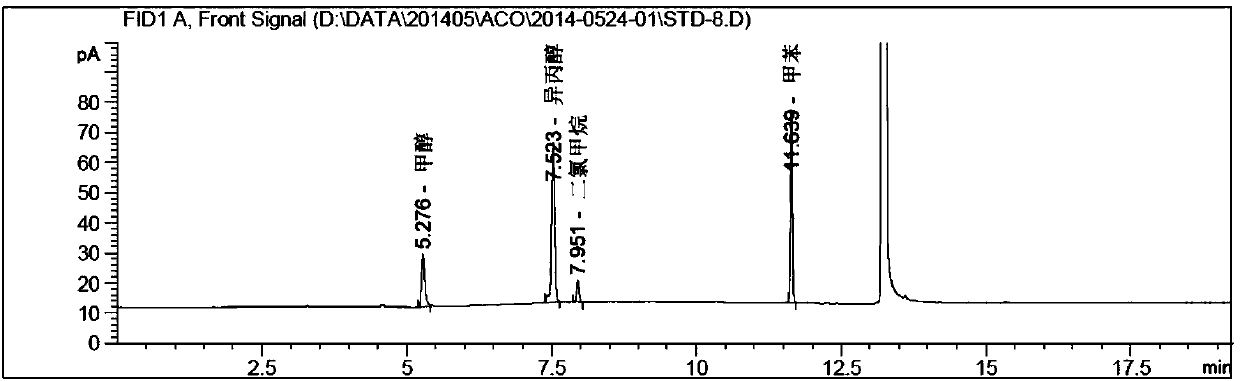
[0094] Instrument: Agilent 7890A-7697A headspace gas chromatograph, FID detector;
[0095] Chromatographic column: DB-624 (30m*0.53mm, 3.0μm);
[0096] Chromatographic parameters: carrier gas: nitrogen; carrier gas flow rate: 2.0ml / min; split ratio: 1:1;
[0097] Detector temperature: 260°C; Injection port temperature: 200°C;
[0098] Column temperature: the initial temperature is 35°C and kept for 5 minutes, then raised to 220°C at a rate of 20°C / min, and kept for 5 minutes.
[0099] Headspace detection parameters: heating box temperature: 80°C; quantitative loop temperature: 100°C; transfer line temperature: 110°C;
[0100] Headspace equilibration time: 20min; Cycle time: 30min; Injection time: 1.0min;
[0101] Pressure balance time: 0.1min.
[0102] experiment procedure:
[0103] Blank solution: dimethylsulfoxide (DMSO).
[0104] System suitability solution: Precisely pipette 1.0ml of each stock solution in Example 1 into a 50ml volumetric flask filled with about 30ml...
Embodiment 3
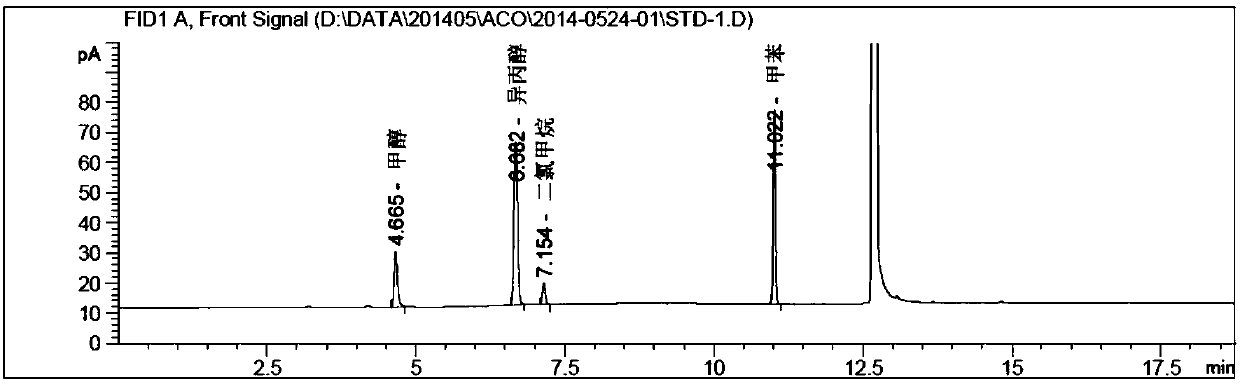
[0109] Instrument: Agilent 7890A-7697A headspace gas chromatograph, FID detector;
[0110] Chromatographic column: DB-624 (30m*0.53mm, 3.0μm);
[0111] Chromatographic parameters: carrier gas: nitrogen; carrier gas flow rate: 2.0ml / min; split ratio: 1:1;
[0112] Detector temperature: 260°C; Injection port temperature: 200°C;
[0113] Column temperature: the initial temperature is 45°C and kept for 5 minutes, then raised to 220°C at a rate of 20°C / min, and kept for 5 minutes.
[0114] Headspace detection parameters: heating box temperature: 80°C; quantitative loop temperature: 100°C; transfer line temperature: 110°C;
[0115] Headspace equilibration time: 20min; Cycle time: 30min; Injection time: 1.0min;
[0116] Pressure balance time: 0.1min.
[0117] experiment procedure:
[0118] Blank solution: dimethylsulfoxide (DMSO).
[0119] System suitability solution: Precisely pipette 1.0ml of each positioning solution in Example 1 into a 50ml volumetric flask filled with about ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com