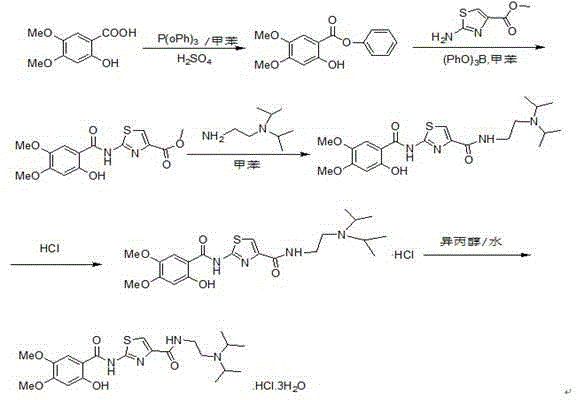
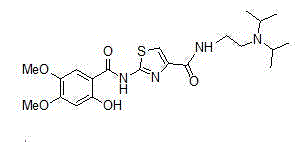
Preparation methods for acotiamide and hydrochloride thereof
A technology of hydrochloric acid and propylethylenediamine, which is applied in the direction of organic chemistry, can solve the problems of slow reaction and incomplete reaction of toluene, and achieve the effect of complete reaction, reduction of toluene residue and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 2-hydroxy-4,5-dimethoxybenzoic acid [Formula (2)] - Reaction Condition 1
[0033] Put 2-iodo-4,5-dimethoxybenzoic acid [Formula (1)] (20.0g), anhydrous copper sulfate (1.0g), 10% sodium hydroxide aqueous solution (300ml), pyridine into a 250ml reaction bottle (4.0ml), under nitrogen protection, stir and heat up to 95-100°C, keep warm for 1.5-2h, take samples for testing, after the reaction is complete (if the reaction is not complete, continue to keep warm for 0.5h), cool down to 20-30°C, filter and collect For the filtrate, add 6N hydrochloric acid (120ml) dropwise to the filtrate under stirring, cool down to 15-25°C, keep warm and stir to crystallize for 0.5-1h, filter, wash the filter cake with 100ml of water, drain it, and dry it with air at 70-80°C 3 After ~4h, the weight was constant, and the material was collected to obtain 12.8g of 2-hydroxy-4,5-dimethoxybenzoic acid.
Embodiment 2
[0035] Preparation of 2-hydroxy-4,5-dimethoxybenzoic acid [Formula (2)] - Reaction Condition 2
[0036] Put 2-iodo-4,5-dimethoxybenzoic acid [Formula (1)] (45.0g), anhydrous copper sulfate (2.3g), 10% sodium hydroxide aqueous solution (675ml), pyridine into a 250ml reaction bottle (10.0ml), under nitrogen protection, stir and heat up to 90°C, keep warm for 2-2.5 hours, take samples for testing, after the reaction is complete (if the reaction is not complete, continue to keep warm for 0.5h), cool down to 20-30°C, filter, and collect the filtrate , add 6N hydrochloric acid (270ml) dropwise to the filtrate under stirring, cool down to 15-25°C, keep stirring and crystallize for 0.5-1h, filter, wash the filter cake with 100ml of water, drain, and dry the material with air at 75-85°C for 2.5~ After 3 hours, the weight was constant, and the material was collected to obtain 27.4 g of 2-hydroxy-4,5-dimethoxybenzoic acid.
Embodiment 3
[0038] Preparation of phenyl 2-hydroxy-4,5-dimethoxybenzoate [Formula (3)]
[0039]Add 2-hydroxy-4,5-dimethoxybenzoic acid [Formula (2)] (11.8g), triphenyl phosphite (20g,), toluene (23ml) into a 250ml reaction bottle, under nitrogen protection, stir Add sulfuric acid (0.6ml), raise the temperature to 110-115°C, keep it warm for 3.5-4.5h, take a sample and check it, after the reaction is complete (if the reaction is not complete, continue to keep warm for 0.5h), cool down to 20-30°C, add methanol (70ml ), continue to cool to 15-25°C, keep warm and crystallize for 0.5-1h, filter, rinse the filter cake with 20ml of methanol, drain it, dry it with air at 40°C to constant weight, and collect the material to obtain 2-hydroxy-4,5 - 11.4 g of phenyl dimethoxybenzoate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com