Acotiamide hydrochloride membrane controlled slow release preparation and preparation method thereof
A technology for acotiamide hydrochloride and sustained-release preparations, which is applied in the field of acotiamide hydrochloride film-controlled sustained-release preparations and its preparation, can solve the problems of easy forgetting and poor compliance, and achieve the goal of improving solubility and dissolution rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
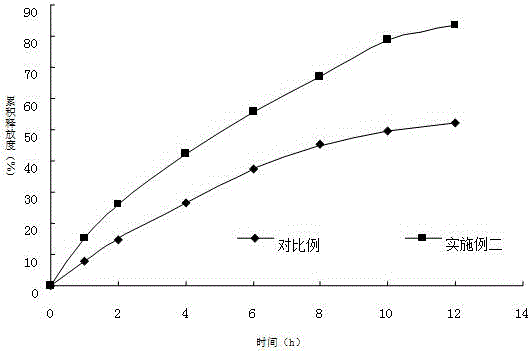
Image
Examples
Embodiment 1
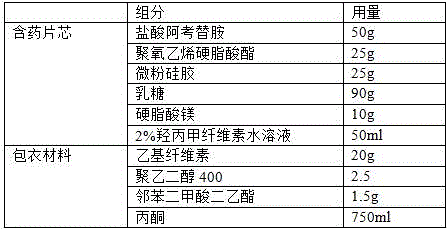
[0018] A film-controlled sustained-release preparation of acotiamide hydrochloride is composed of highly soluble acotiamide hydrochloride intermediates and coating components; it includes the following raw materials in parts by weight:
[0019] Table 1 Formula for preparing 1000 drug-containing tablet cores
[0020]
[0021] Its preparation process is:
[0022] (1) Preparation of highly soluble acotiamide hydrochloride intermediate: Dissolve acotiamide hydrochloride in 2.5 times the volume of ethanol solution, add polyoxyethylene stearate melted in a water bath at 60~70°C, mix and stir Evenly, dry in a vacuum oven at 40°C for 24 hours;
[0023] (2) Preparation of drug-containing tablet cores: Take out the highly soluble acotiamide hydrochloride intermediate obtained in step (1), add the prescribed amount of micropowder silica gel to grind (micropowder silica gel as a lubricant can reduce the loss of solid dispersion during the grinding process ), passed through an 80-mesh...
Embodiment 2
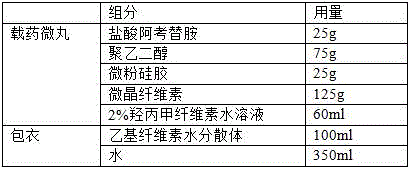
[0025] A film-controlled sustained-release preparation of acotiamide hydrochloride is composed of highly soluble acotiamide hydrochloride intermediates and coating components; it includes the following raw materials in parts by weight:
[0026] Table 2 The formula for preparing 1000 drug-loaded pellets
[0027]
[0028] Its preparation process is:
[0029] (1) Preparation of highly soluble acotiamide hydrochloride intermediate: Dissolve acotiamide hydrochloride in 1.5 times the volume of ethanol solution, add polyethylene glycol melted in a water bath at 60~70°C, mix and stir evenly, and place Dry in a vacuum oven at 40°C for 24 hours;
[0030] (2) Preparation of drug-loaded pellets: Take out the highly soluble acotiamide hydrochloride intermediate obtained in step (1), add the prescribed amount of micropowder silica gel to grind, pass through an 80-mesh sieve, and mix with lactose, microcrystalline cellulose, etc. Mix evenly by incremental method, add appropriate amount ...
Embodiment 3
[0032] A film-controlled sustained-release preparation of acotiamide hydrochloride is composed of highly soluble acotiamide hydrochloride intermediates and coating components; it includes the following raw materials in parts by weight:
[0033] Table 3 Formula for preparing 1000 drug-loaded pellets
[0034]
[0035] Its preparation process is:
[0036] Add an appropriate amount of microcrystalline cellulose to the binder to make a soft material, and use the extrusion spheronization method to prepare a blank pellet core (or directly use a commercially available blank pellet core); dissolve acotiamide hydrochloride in 1 volume of ethanol In the solution, after the polyethylene glycol is heated in a water bath at 60~70°C until it melts, mix and stir evenly, add 2% hypromellose aqueous solution and stir evenly, adopt the fluidized bed coating method, and dissolve the medicinal solution Spray on the surface of the blank pellet core to prepare drug-loaded pellets; after drying, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com