Acotiamide hydrochloride sustained release tablet and preparation method thereof
A technology of acotiamide hydrochloride and acotiamide hydrochloride trihydrate, applied in the field of acotiamide hydrochloride sustained-release tablet and its preparation, can solve the problem of poor compliance, large difference in release degree, waste of manpower and material resources, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
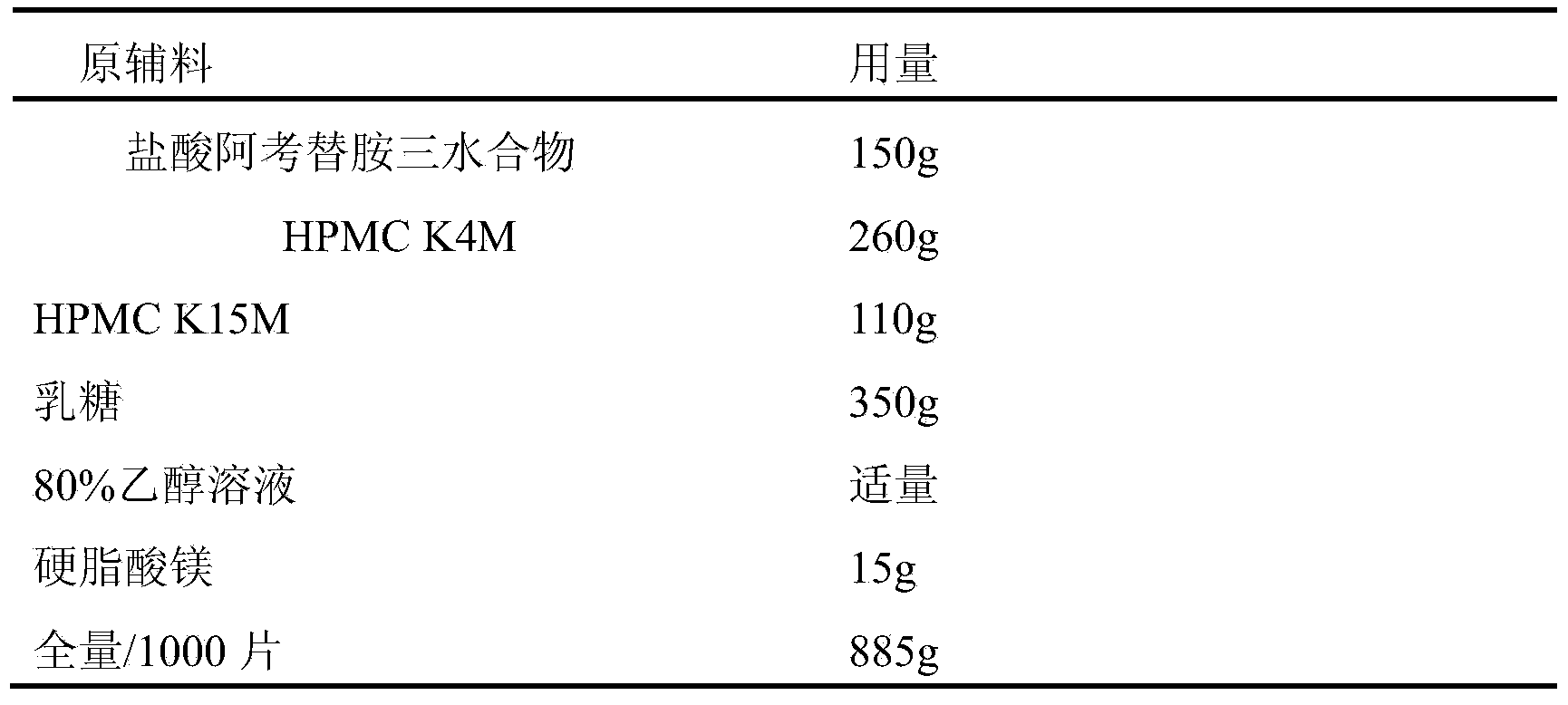
[0031] 1. Prescription
[0032] plain tablet prescription
[0033] After various prescription process research, according to the common ratio of various excipients, the prescription of plain tablets is determined as
[0034] Acotiamide Hydrochloride Sustained Release Tablets Prescription
[0035]
[0036] Coating prescription
[0037] Acotiamide Hydrochloride Sustained Release Tablets Coated Prescription 1000 Tablets
[0038]
[0039]
[0040] 2. Preparation process
[0041] Take acotiamide hydrochloride trihydrate, pulverize it and pass through an 80-mesh sieve, stir it with lactose in a high-speed mixer for 10 minutes, moisten it with 80% ethanol and granulate it, dry it at 50-55°C, and control the moisture content of the granules after drying When it is less than 0.5%, the granules pass through a 40-mesh sieve for granulation, add HPMC K4M, HPMC K15M, magnesium stearate and mix evenly, and then press into tablets. Controlled at 7-8kg, the prepared plain tablet...
Embodiment 2
[0053] 1. Prescription
[0054] plain tablet prescription
[0055] After various prescription process research, according to the common ratio of various excipients, the prescription of plain tablets is determined as
[0056] Acotiamide Hydrochloride Sustained Release Tablets Prescription
[0057]
[0058] Coating prescription
[0059] Acotiamide Hydrochloride Sustained Release Tablets Coated Prescription 1000 Tablets
[0060]
[0061] 2. Preparation process
[0062] Take acotiamide hydrochloride trihydrate, pulverize it and pass through an 80-mesh sieve, stir it with lactose in a high-speed mixer for 10 minutes, moisten it with 80% ethanol and granulate it, dry it at 50-55°C, and control the moisture content of the granules after drying When it is less than 0.5%, the granules pass through a 40-mesh sieve for granulation, add HPMC K4M, HPMC K15M, magnesium stearate and mix evenly, and then press into tablets. Controlled at 7-8kg, the prepared plain tablets were coated ...
Embodiment 3
[0073] Embodiment 3 stability study result
[0074] high temperature test
[0075] Take this product (Example 1 (100,000 pieces of pilot test product), batch number: 20130709), put it in a constant temperature drying oven at 60°C for 10 days, and take samples on the 5th and 10th days respectively. , Dissolution, related substances and labeling quantities and other inspection items were measured, and the results are shown in the table below:
[0076]
[0077] High Humidity Test
[0078] Take this product (Example 1 (100,000 pieces of pilot test product), batch number: 20130709), place it in a constant humidity airtight desiccator, and place it at 25°C and a relative humidity of 92.5% for 10 days. Samples were taken on the first 10 days, and the inspection items such as properties, moisture, dissolution rate, polymers, related substances and labeled quantities were measured. The results are shown in Table 14-2:
[0079]
[0080] Strong light exposure test
[0081] Take...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com