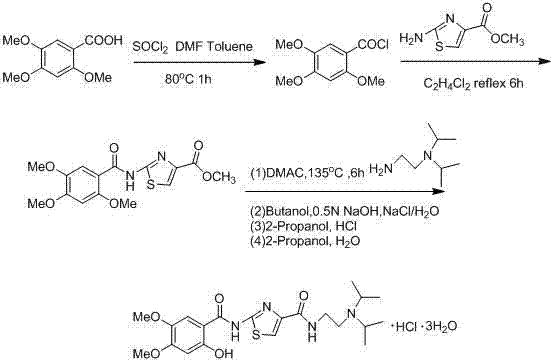
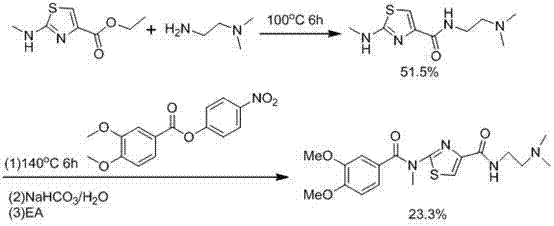
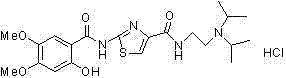Synthetic method of acotiamide hydrochloride
A technology of acotiamide hydrochloride and a synthesis method, which is applied in the synthesis field of acotiamide hydrochloride, can solve the problems of many side reactions, poor reaction selectivity, high toxicity, etc., and achieves less operation steps, less side reactions, and high selectivity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 2-tert-butyldimethylsiloxy-4,5-dimethoxybenzoic acid (2a)
[0048] Dissolve 2-hydroxy-4,5-dimethoxybenzoic acid (100g) in anhydrous toluene (400ml), slowly add TBSOTf (160g) to the solution at room temperature, stir for 5 hours, add purified water to wash To neutrality, take the organic phase, add anhydrous sodium sulfate (20 g) and dry for 8 hours, filter, and evaporate to dryness under reduced pressure to obtain 134 g of the title compound, yield 85%, melting point: 235°C
[0049] H1-NMR(DMSO, 400 MHz) δ: 0.08(s, 3H,-SiCH3), 0.09(s, 3H,-SiCH3), 0.14(s, 3H, -CH3); 0.22(s, 3H, -CH3) , 0.35(s,3H, -CH3), 4.59(s, 3H,-CH3), 4.68(s, 3H,-CH3), 7.26(s, 1H,ArH), 7.63(s, 1H,ArH), 11.27 (s, 1H, COOH).
Embodiment 2
[0051] Synthesis of 2-tert-Butoxycarbonyl-4,5-dimethoxybenzoic acid (2b)
[0052] Dissolve 2-hydroxy-4,5-dimethoxybenzoic acid (100g) in anhydrous toluene (400ml), add Boc2O (132g) at room temperature and stir at room temperature for 3 hours, add 10% citric acid aqueous solution (100ml) washed three times, washed with purified water until neutral, added with anhydrous sodium sulfate (20g) and dried for 8 hours, filtered and evaporated to dryness under reduced pressure to obtain the title compound (135g), yield 90%, melting point: 190℃
[0053] H1-NMR(DMSO, 400 MHz) δ: 1.34(s,3H), 1.37(s,3H), 1.40(s,3H), 3.77(s,3H), 3.82(s, 3H), 7.17(s, 1H), 7.50(s, 1H), 13.45-13.70(bs, 1H).
Embodiment 3
[0055] Step 1: Preparation of 2-[N-(2-tert-butyldimethylsilyloxy-4,5-dimethoxybenzoyl)amino]-4-methoxycarbonyl-1,3-thiazole
[0056] Dissolve 2-hydroxy-4,5-dimethoxybenzoic acid (100g) in anhydrous toluene (400ml), slowly add TBSOTf (160g) to the solution at room temperature, stir for 5 hours, add purified water to wash To neutrality, take the organic phase, add anhydrous sodium sulfate (20g) and dry for 8 hours, filter, add thionyl chloride (60g) and N,N-dimethylformamide (0.18ml) to the filtrate, Stir at 80°C for 4 hours, add 2-amino-4-methoxycarbonyl-1,3-thiazole (80g) to the compound, stir at 100°C for 5 hours, after the reaction is over, cool to room temperature, and filter the precipitate Crystals, crystals were added to 1.6 liters of water, 400 grams of ice was added to stir, and 10% by mass sodium hydroxide aqueous solution was added, the pH of the aqueous solution was adjusted to 7.5, and then stirred at room temperature for 3 hours. The crystals were collected by filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com