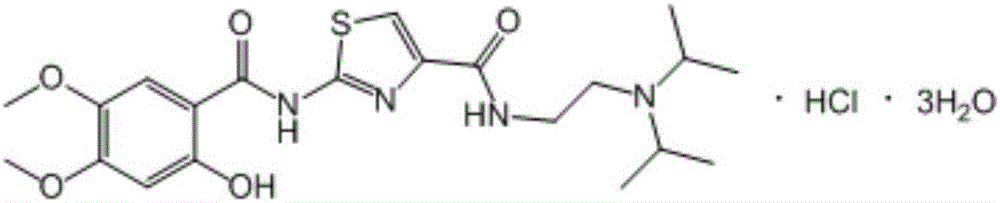
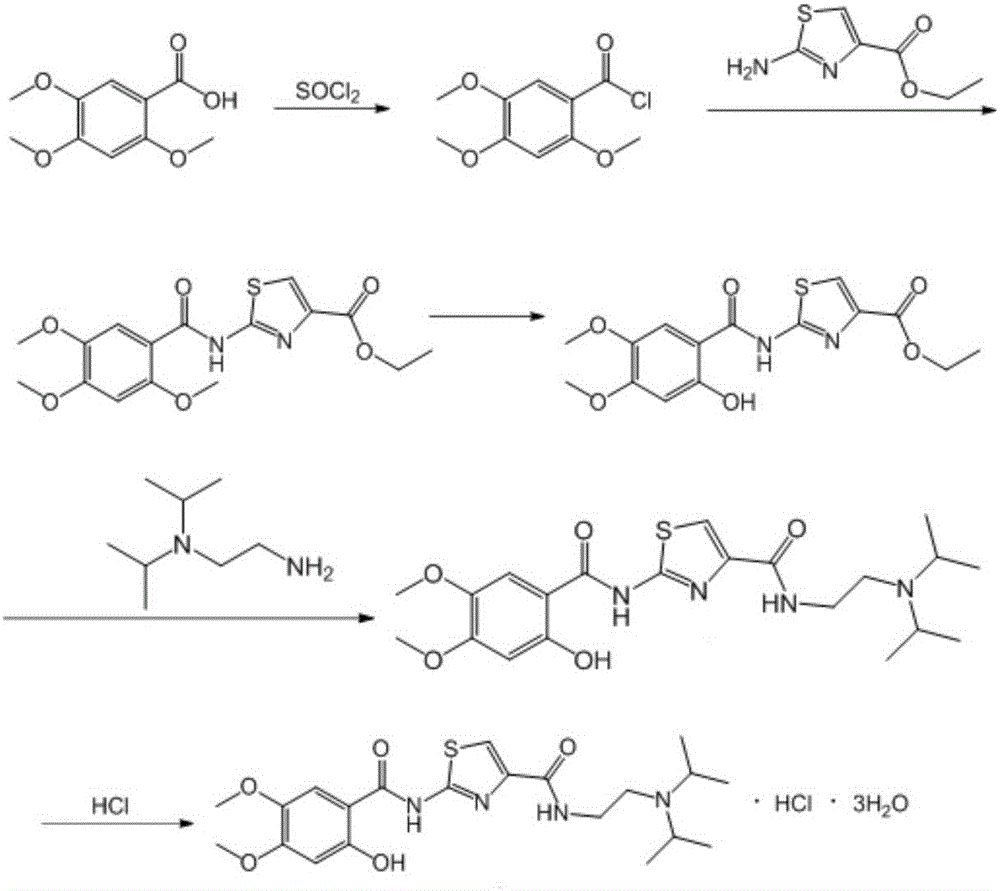
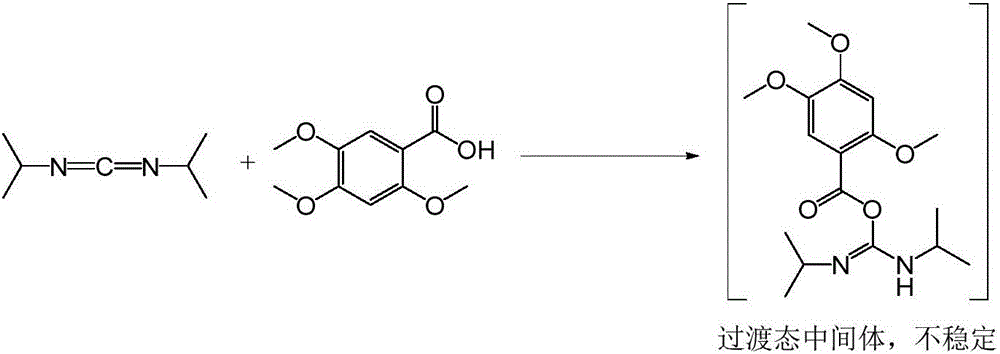
Acotiamide hydrochloride intermediate, and synthesis technique and application thereof
A technology of acotiamide hydrochloride and synthesis process, applied in the direction of organic chemistry and the like, can solve the problems of complicated post-processing, unsuitable for industrial scale-up production, long reaction time, etc., and achieves the advantages of short reaction time and reduction of rearrangement to generate by-products. The effect of less chance and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of the acotiamide hydrochloride intermediate 2-[N-(2,4,5-trimethoxybenzoyl)amino]-4-ethoxycarbonyl-1,3-thiazole provided in this example includes the following steps step:
[0032]
[0033] 25.5g (0.12mol) 2,4,5-trimethoxybenzoic acid, 22.7g (0.132mol) ethyl 2-aminothiazole-4-carboxylate, 17.8g (0.132mol) 1-hydroxybenzotriazole , 20mL of triethylamine and 500mL of dichloromethane were put into a 1L reaction flask, cooled to 5°C, and 30mL of dichloromethane solution dissolved with 18.0g (0.143mol) of diisopropylcarbodiimide was added dropwise under stirring, and the dropwise , heated to 30°C for 4 hours, filtered, washed the filter cake with dichloromethane, and dried in vacuo to obtain 27.5g of 2-[N-(2,4,5-trimethoxybenzoyl)amino]-4-ethane Oxycarbonyl-1,3-thiazole, the yield is 62.5%, and the purity of the product measured by HPLC normalization method is 98.8%.
Embodiment 2
[0035] The preparation method of the acotiamide hydrochloride intermediate 2-[N-(2,4,5-trimethoxybenzoyl)amino]-4-ethoxycarbonyl-1,3-thiazole provided in this example includes the following steps step:
[0036]
[0037] 51g (0.24mol) 2,4,5-trimethoxybenzoic acid, 44.7g (0.26mol) ethyl 2-aminothiazole-4-carboxylate, 35.6g (HOBt, 0.26mol) 1-hydroxybenzotriazepam Put oxazole, 50mL N,N-diisopropylethylamine and 600mL 1,2-dichloroethane into a 1L reaction flask, cool to 10°C, add dropwise 40.0g (0.32mol) diisopropyl 50 mL of carbodiimide in 1,2-dichloroethane solution was added, and the temperature was raised to 50° C. to react for 3 hours. Filtration, the filter cake was soaked with 1,2-dichloroethane, and vacuum-dried to obtain 58g 2-[N-(2,4,5-trimethoxybenzoyl)amino]-4-ethoxycarbonyl-1, 3-thiazole, the yield is 65.9%, and the purity of the product measured by HPLC normalization method is 97.6%.
Embodiment 3
[0039] The preparation method of the acotiamide hydrochloride intermediate 2-[N-(2,4,5-trimethoxybenzoyl)amino]-4-ethoxycarbonyl-1,3-thiazole provided in this example includes the following steps step:
[0040]
[0041]25.5g (0.12mol) 2,4,5-trimethoxybenzoic acid, 20.9g (0.1212mol) ethyl 2-aminothiazole-4-carboxylate, 24.27g (0.18mol) 1-hydroxybenzotriazole , 17.5mL of triethylamine and 500mL of dichloromethane were put into a 1L reaction flask, cooled to 0°C, and 50mL of dichloromethane solution in which 45.3g (0.35mol) of diisopropylcarbodiimide was dissolved was added dropwise under stirring. After completion, the temperature was raised to 35°C for 2.5 hours, filtered, the filter cake was soaked with dichloromethane, and vacuum-dried to obtain 29.9 g of 2-[N-(2,4,5-trimethoxybenzoyl)amino]-4 -Ethoxycarbonyl-1,3-thiazole, the yield is 68%, and the purity of the product measured by HPLC normalization method is 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com