Acotiamide hydrochloride dihydrate crystal, and preparation method and applications thereof
A technology of acotiamide hydrochloride and dihydrate, which is applied to the crystal form of acotiamide hydrochloride dihydrate and the field of preparation thereof, and can solve the problem that no literature mentions the crystal form of acotiamide hydrochloride dihydrate and its preparation. Preparation method, no mention of crystal form spectral data and drug application, etc., to achieve the effects of stable chemical properties, stable properties and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Add 20.0 g of acotiamide hydrochloride trihydrate and 200 mL of ethanol into the reaction flask, raise the temperature to reflux, stir for 30 min, and naturally cool to room temperature with stirring to crystallize, filter, and dry the filter cake at 60 ° C to obtain 16.6 g of hydrochloric acid Acotiamide dihydrate has a yield of 83.0% and a purity of 99.86%.
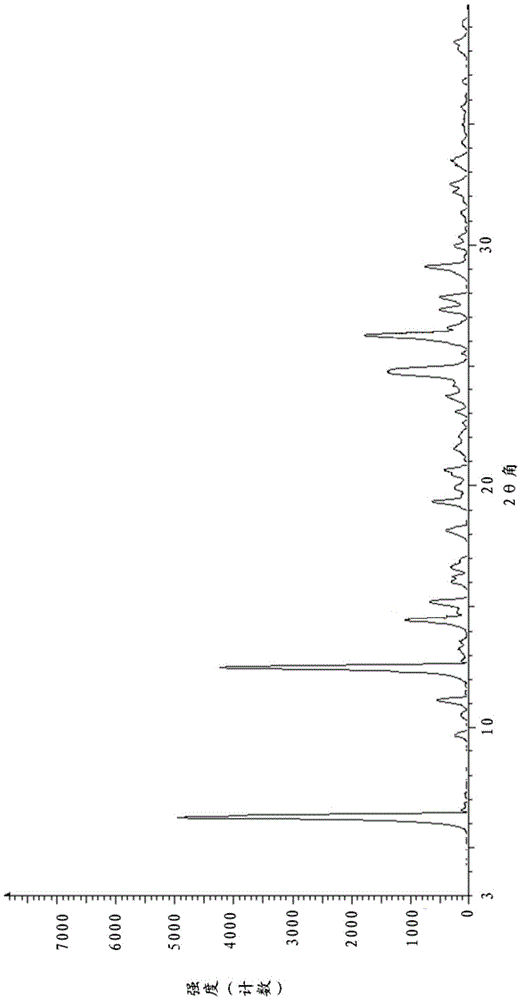
[0070] Measure the X-ray powder diffraction pattern, such as figure 1 The detailed parameters are shown in Table 2, showing the position of the diffraction peak: 2θ value (°); the relative intensity of the diffraction peak: peak height (Height%).
[0071] The X-ray diffraction parameter of the crystal form of acotiamide hydrochloride dihydrate of table 2
[0072]
[0073]
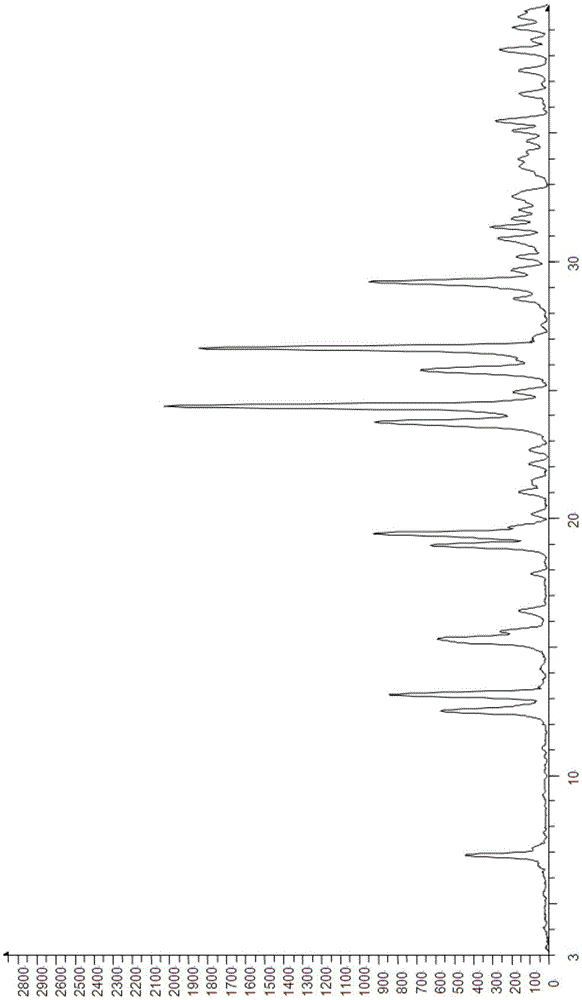
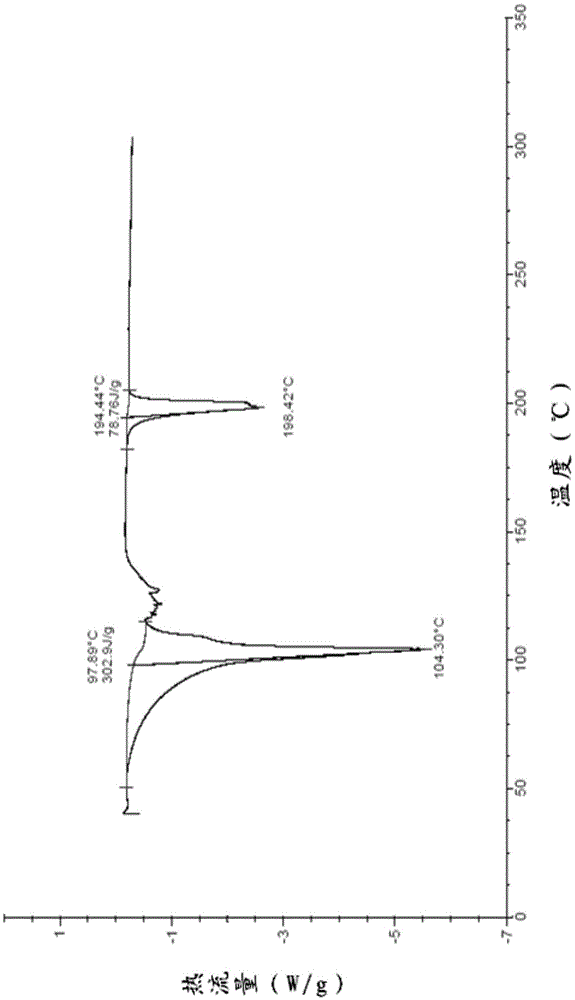
[0074] Differential thermal analysis and thermogravimetric analysis are carried out to the prepared acotiamide hydrochloride dihydrate, wherein the DCS collection of illustrative plates is as follows image 3 , TG spectrum such as Fi...
Embodiment 2
[0078] The stability of acotiamide hydrochloride dihydrate obtained in Example 1 was investigated, and acotiamide hydrochloride trihydrate was used as a contrast. The test results are shown in Table 3:
[0079] Table 3 Stability test results of acotiamide hydrochloride dihydrate prepared in Example 1 of the present invention
[0080]
[0081] The test results show that the crystal form of acotiamide hydrochloride dihydrate of the present invention has stable physical and chemical properties, has no obvious hygroscopicity in the influencing factor test and accelerated test, and the crystal form does not change.
Embodiment 3
[0083] Investigate the solubility of acotiamide hydrochloride dihydrate crystal form of the present invention, measure the solubility of acotiamide hydrochloride dihydrate in a series of buffer solutions within the physiological pH range, use acotiamide hydrochloride trihydrate crystal Type is control, and the test results are shown in Table 4:
[0084] Table 4 Solubility Test Results
[0085]
[0086] The test results show that the dihydrate crystal form of acotiamide hydrochloride of the present invention is compared with the original marketed acotiamide hydrochloride trihydrate crystal form, and the solubility in three pH ranges in the two media is similar, indicating that the dihydrate crystal form The solubility is better than that of the trihydrate crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com