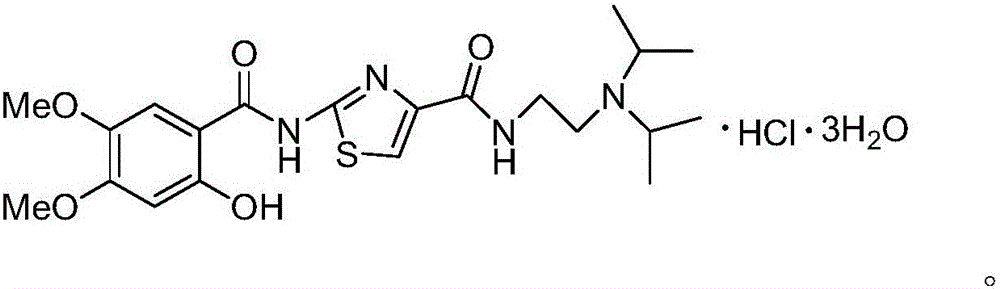
Acotiamide hydrochloride trihydrate refining method and acotiamide hydrochloride trihydrate preparation method
A technology for acotiamide hydrochloride and acotiamide hydrochloride sodium salt, which is applied in the field of refining and preparation of acotiamide hydrochloride trihydrate, and can solve problems such as difficulty in removal, impact on product quality, and poor removal of impurities , achieve the effect of reducing cost and improving the purity of liquid phase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
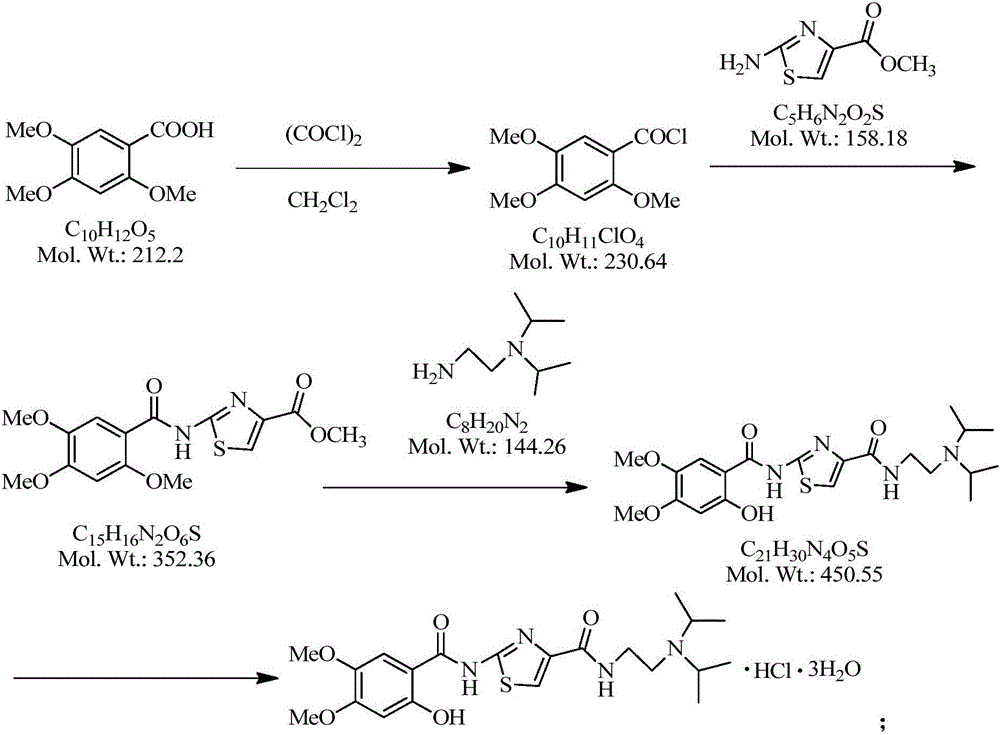
[0035] The preparation method of acotiamide and acotiamide hydrochloride:
[0036] (1) The preparation method of acotiamide:
[0037] 2-[(2,4,5-trimethoxybenzoyl)amino]-1,3-thiazole-4-carboxylic acid methyl ester was prepared by prior art, and then 2-[(2,4,5- Methyl trimethoxybenzoyl)amino]-1,3-thiazole-4-carboxylate was suspended in DMAC (dimethylacetamide), N,N-diisopropylethylenediamine was added, under nitrogen protection , heat up to 130-135°C, start timing when the reaction liquid is fully dissolved, turn off the heating after reacting for about 20 hours, drop to 20-30°C, add drinking water to dilute, then add ethyl acetate to wash 3 times, combine ethyl acetate and drinking water Back-extract twice, combine the water layers and evaporate to dryness under reduced pressure at 75-80°C, add drinking water, stir at 20-30°C for 16 hours, filter, and dry under reduced pressure at 50°C for 20 hours to obtain off-white solid N-[2-(diisopropyl Amino)ethyl]-2-[(2-hydroxy-4,5-dim...
Embodiment 1
[0059] Preparation of acotiamide:
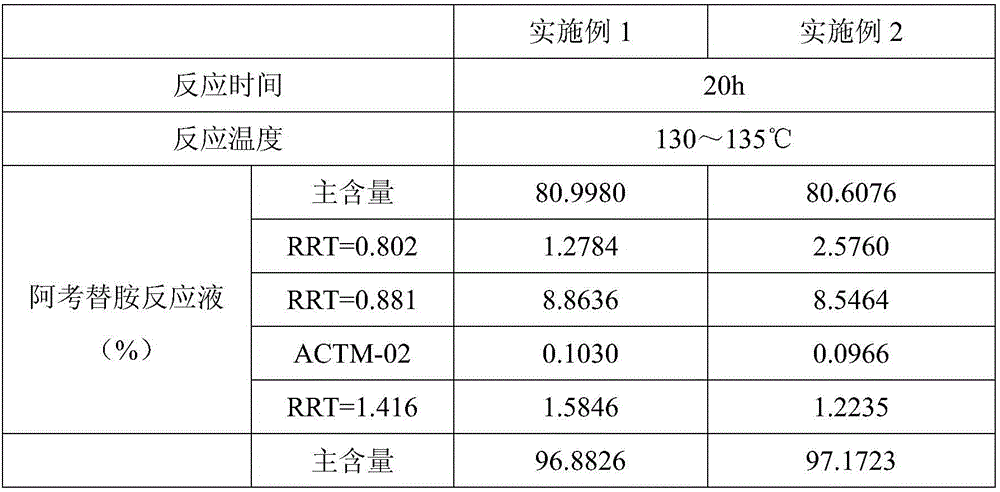
[0060] Suspend 20g of 2-[(2,4,5-trimethoxybenzoyl)amino]-1,3-thiazole-4-carboxylic acid methyl ester (ACTM-02 for short) in 40ml of DMAC (dimethylacetamide) , add 18.8g N,N-diisopropylethylenediamine (ACTS-03 for short), under the protection of nitrogen, heat up to 130-135°C, start timing when the reaction solution is completely dissolved, turn off the heating after about 20 hours of reaction, and drop to 20- 30°C, add 200ml of water to dilute, add 70ml of ethyl acetate to wash 3 times, combine ethyl acetate and 70ml of water for stripping twice, combine the water layer and evaporate to dryness under reduced pressure at 75-80°C, add 60g of water and stir at 20-30°C for 16h , filtered, and dried under reduced pressure at 50° C. for 20 h to obtain 18.4 g of an off-white solid (ie, the crude product of acotiamide), with a yield of 72%.
[0061] Refined (acotiamide into acotiamide sodium salt):
[0062] Add 15.0g of crude acotiamide to a 250ml...
Embodiment 2
[0066] Preparation of acotiamide:
[0067] Suspend 20g of 2-[(2,4,5-trimethoxybenzoyl)amino]-1,3-thiazole-4-carboxylic acid methyl ester (ACTM-02 for short) in 40ml of DMAC (dimethylacetamide) , add 18.8g N,N-diisopropylethylenediamine (ACTS-03 for short), under the protection of nitrogen, heat up to 130-135°C, start timing when the reaction solution is completely dissolved, turn off the heating after about 20 hours of reaction, and drop to 20- 30°C, add 200ml of water to dilute, add 70ml of ethyl acetate to wash 3 times, combine ethyl acetate and 70ml of water for stripping twice, combine the water layer and evaporate to dryness under reduced pressure at 75-80°C, add 60g of water and stir at 20-30°C for 16h , filtered, and dried under reduced pressure at 50° C. for 20 h to obtain 19.6 g of off-white solid with a yield of 77%.
[0068] Refined (acotiamide into acotiamide sodium salt):
[0069] Add 15.0g crude acotiamide to a 250ml three-neck flask, stir 90ml0.5mol / L potassiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com