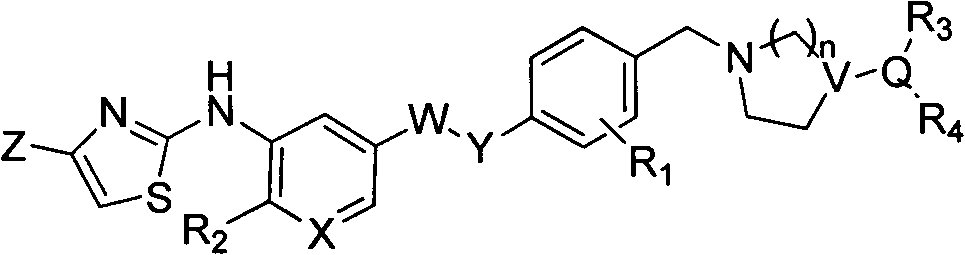
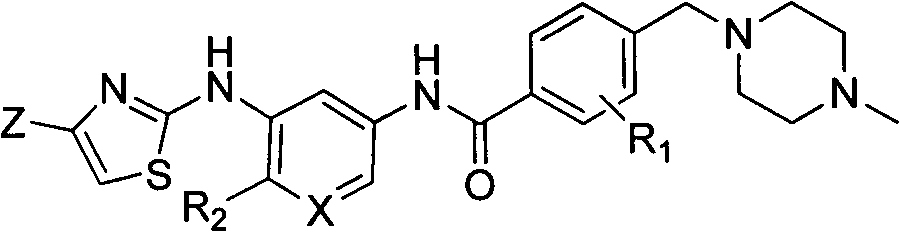
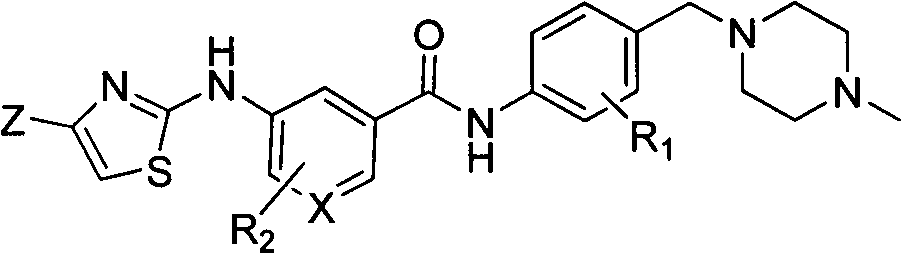
2-aminothiazoles compound
A compound, alkyl technology, applied in the field of application in the preparation of drugs, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1: N-{4-[(4-methylpiperazinyl)methyl]phenyl}-4-methyl-3-[4-(pyridin-3-yl)thiazole-2-amino]benzene Preparation of formamide (reference)
[0140]
[0141] Step A: Preparation of methyl 3-(3-acetylthiourea)-4-methylbenzoate
[0142]
[0143] Ammonium thiocyanate (50 mmol) was dissolved in 50 ml of acetone, a solution of acetyl chloride (50 mmol) in 10 ml of acetone was added dropwise at 40°C, stirred for 1 hour, then cooled to room temperature, and 3-amino-4 - 75 ml of acetone solution of methyl methylbenzoate, stirred at room temperature for 5 hours, added 100 ml of water, continued to stir for 1 hour, filtered the precipitate, washed 3 times with water and 3 times with n-hexane, and dried in vacuo to obtain the title compound 11.3 grams. 1 H-NMR (DMSO), δ12.19(1H, s), 11.56(1H, s), 8.18(1H, s), 7.77(1H, d, J=8.0Hz), 7.45(1H, d, J= 8.0Hz), 3.84(3H, s), 2.27(3H, s), 2.17(3H, s).
[0144] Step B: Preparation of methyl 4-methyl-3-[4-(pyridin-3-yl)thiazole-2...
Embodiment 2
[0153] Example 2: N-{3-trifluoromethyl-4-[(4-methylpiperazinyl)methyl]phenyl}-4-methyl-3-[4-(pyridin-3-yl) Preparation of Thiazole-2-amino]benzamide
[0154]
[0155] According to the synthesis method in Step D of Example 1, the compound in Step C of Example 1 was reacted with 4-[(4-methylpiperazinyl)methyl]-3-trifluoromethylaniline to obtain 20 mg of the title compound. 1 H-NMR (CDCl 3), δ9.61(1H, s), 9.12(1H, s), 8.83(1H, s), 8.49(1H, s), 8.23(1H, d, J=6.8Hz), 7.58(1H, d, J=7.2Hz), 7.51(1H, s), 7.42(1H, s), 7.32(1H, d, J=7.2Hz), 2.36(3H, s).MS: m / z, 567.2(M+H ).
Embodiment 3
[0156] Example 3: N-{4-methyl-3-[4-(6-trifluoromethyl-imidazo[1,2-a]pyridin-3-yl)thiazole-2-amino]phenyl}-3 -Preparation of trifluoromethyl-4-[(4-methylpiperazinyl)methyl]benzamide
[0157]
[0158] Step A: (E)-N,N-Dimethyl-N'-[5-(trifluoromethyl)pyridin-2-yl]formamidine
[0159]
[0160] 5-Trifluoromethyl-2-aminopyridine (20.1 mmol) was dissolved in 10 ml of N,N-dimethylformamide dimethyl acetal, and heated to reflux at 110° C. for 1 hour. The solvent was removed by concentration to obtain 4.2 g of the title compound. 1 H-NMR (CDCl 3 )δ8.52(1H, s), 8.47(1H, s), 7.73(1H, d, J=8Hz), 6.99(1H, d, J=8Hz), 3.14(6H, d, J=8Hz).
[0161] Step B: Preparation of 1-[6-trifluoromethyl-imidazo[1,2-a]pyridin-3-yl]ethanone
[0162]
[0163] The above compound (1 g, 4.6 mmol), sodium iodide (4.4 mmol) and bromoacetone (8.28 mmol) were dissolved in dry N,N-dimethylformamide (5 ml) at 100 Stir at °C for 4 hours. Cool to room temperature, concentrate to remove the solvent, add 20 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com