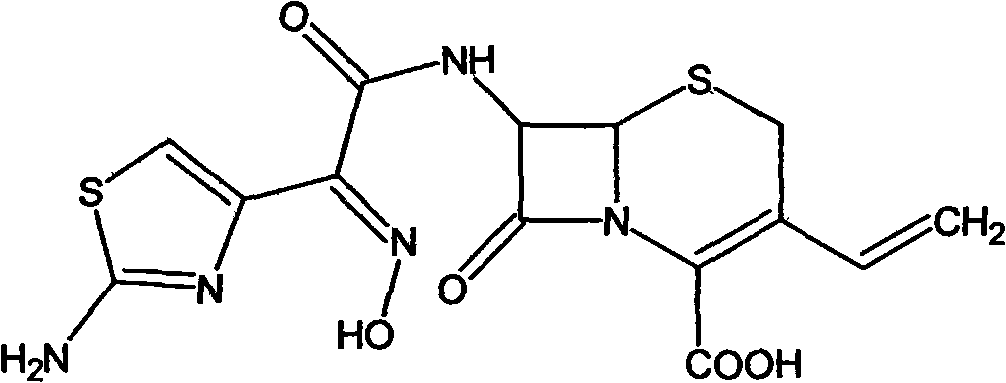
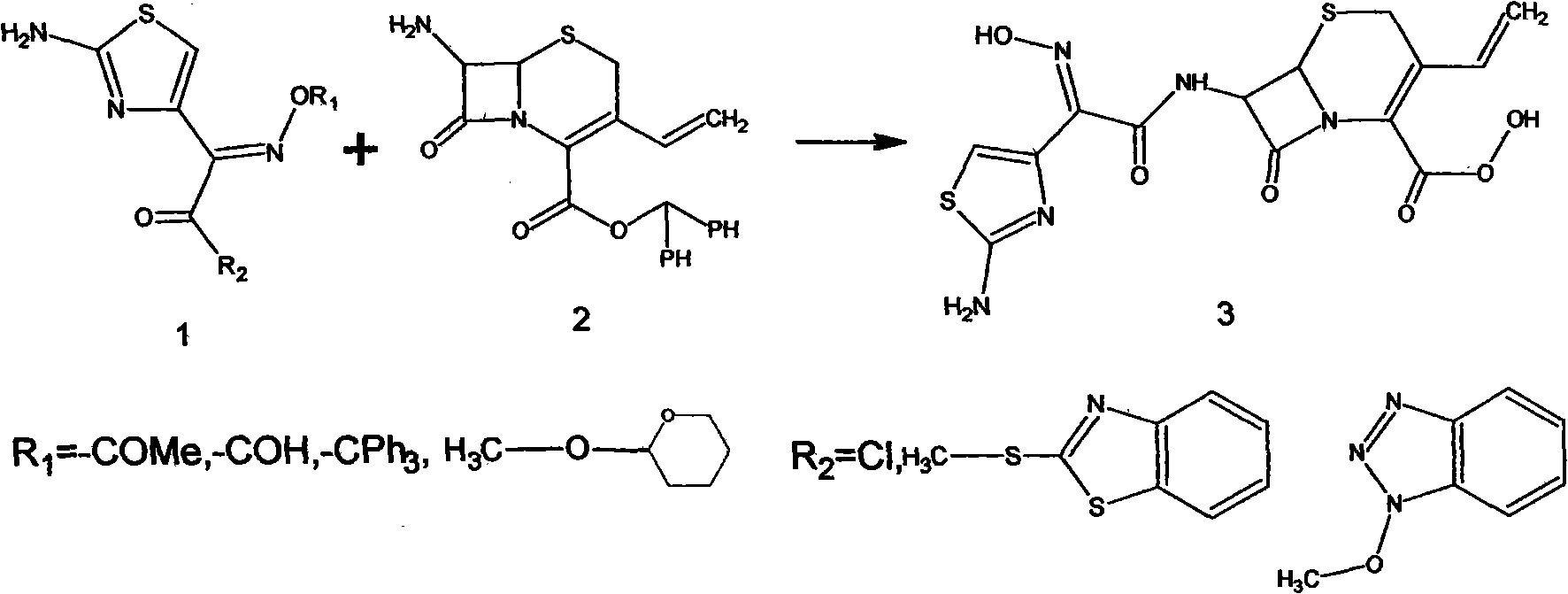
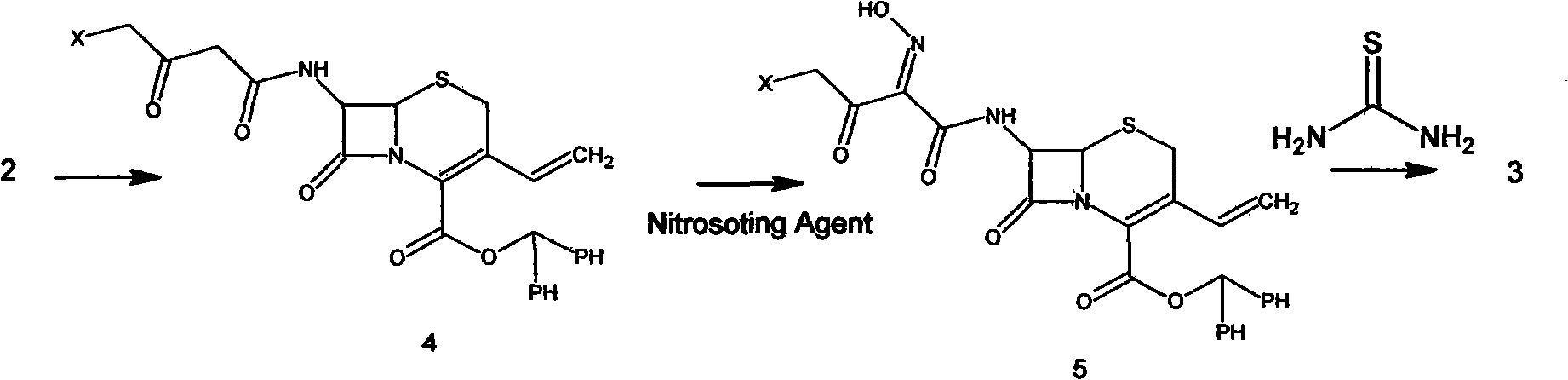
Preparation method of cefdinir
A technology of cefdinir and 7-AVCA, applied in the field of preparation of cefdinir, can solve the problems of product yield and finished product quality influence, cost and environmental pollution, low synthesis yield, etc. The effect of recycling and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] a. Put 20 g of 7-AVCA, 36 g of cefdinir active methyl ester, 100 ml of water, and 200 ml of methanol into a 500 ml three-necked flask, and dropwise add 20 g / 50 ml of tri-n-butylamine / methanol mixture at -10 ° C, and the addition is complete , Insulate and react at -10°C for 24 hours, take samples for HPLC until the 7-AVCA residue is qualified, adjust ph=5 with dilute hydrochloric acid, recover most of the methanol under reduced pressure; add 100ml of water and 200ml of ethyl acetate, stir for 30 minutes, and let it stand for 30 minutes minute. Separate the water layer, add 50ml of water to the oil layer, stir for 30 minutes, and let stand for 30 minutes. The solvent is recovered from the oil layer to obtain the by-product mercaptobenzothiazole. Add 5 g of activated carbon to the combined water layer, decolorize at 25°C for 1 hour, filter, adjust the pH of the filtrate to 3.0 with dilute hydrochloric acid at 25°C, cool down to 5°C and stir for 1 hour, and filter to obta...
Embodiment 2
[0037] Put 20 grams of 7-AVCA, 36 grams of cefdinir active methyl ester, 100 ml of water, and 200 ml of acetone into a 500 ml three-necked flask, add 20 g / 50 ml of tri-n-butylamine / acetone mixture dropwise at -5 ° C, and after the addition, - Incubate at 5°C for 24 hours, take a sample for HPLC until the 7-AVCA residue is qualified, and the post-processing steps are the same as in Example 1.
Embodiment 3
[0039] Put 20 grams of 7-AVCA, 36 grams of cefdinir active methyl ester, 100 ml of water, and 200 ml of ethanol into a 500 ml three-neck flask, add 20 g / 50 ml of triethylamine / ethanol mixture dropwise at 0°C, and drop it at -5°C Incubate the reaction for 24 hours, take a sample for HPLC until the 7-AVCA residue is qualified, and the post-processing steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com