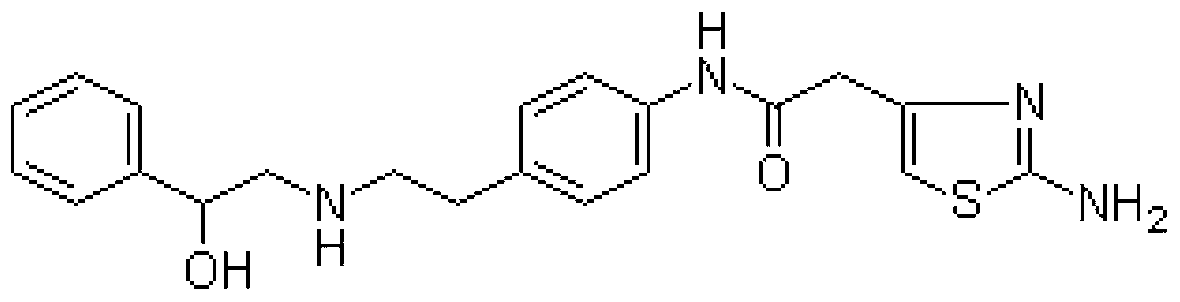
Novel synthesis method of mirabegron
A synthetic method, Mirabegron's technology, applied in the field of chemical drug synthesis, can solve the problems of unfavorable operators and ecological environment, high price, and bad smell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
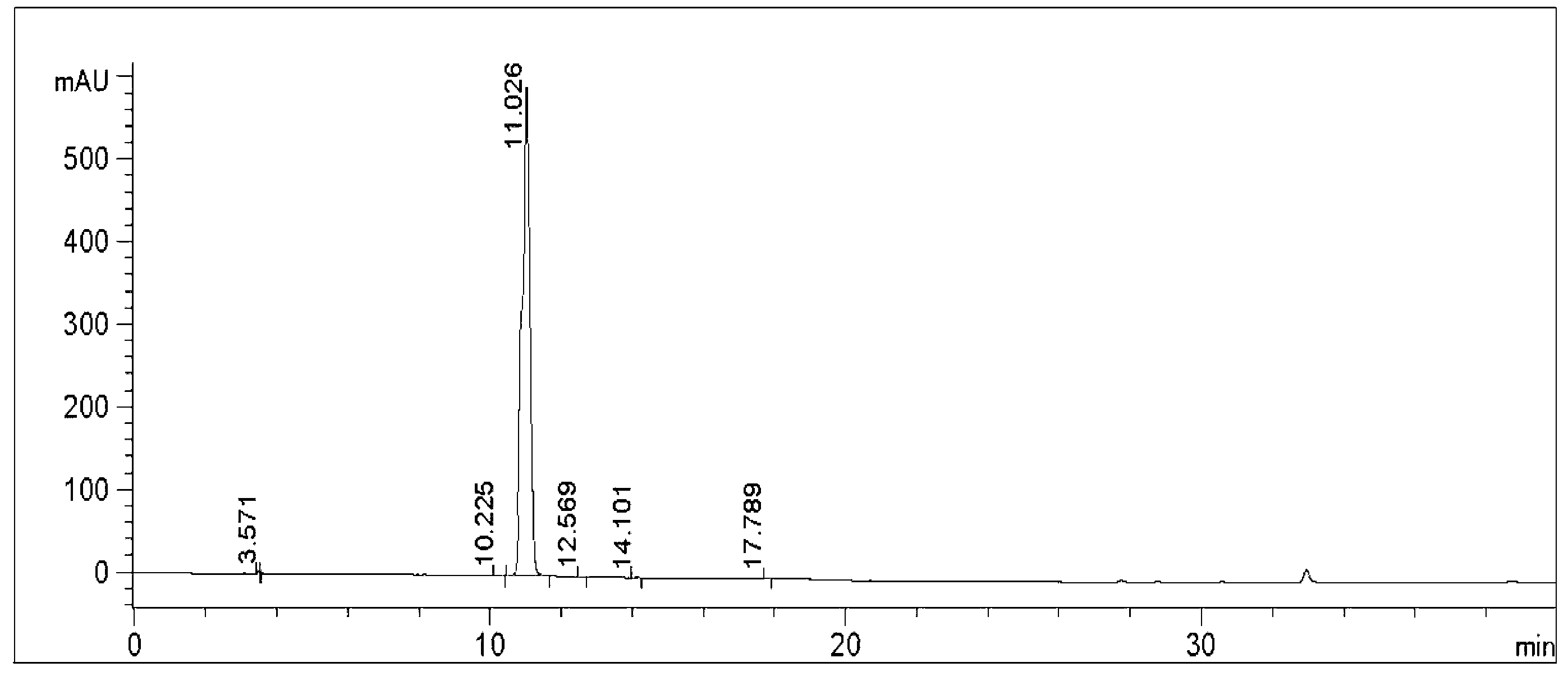
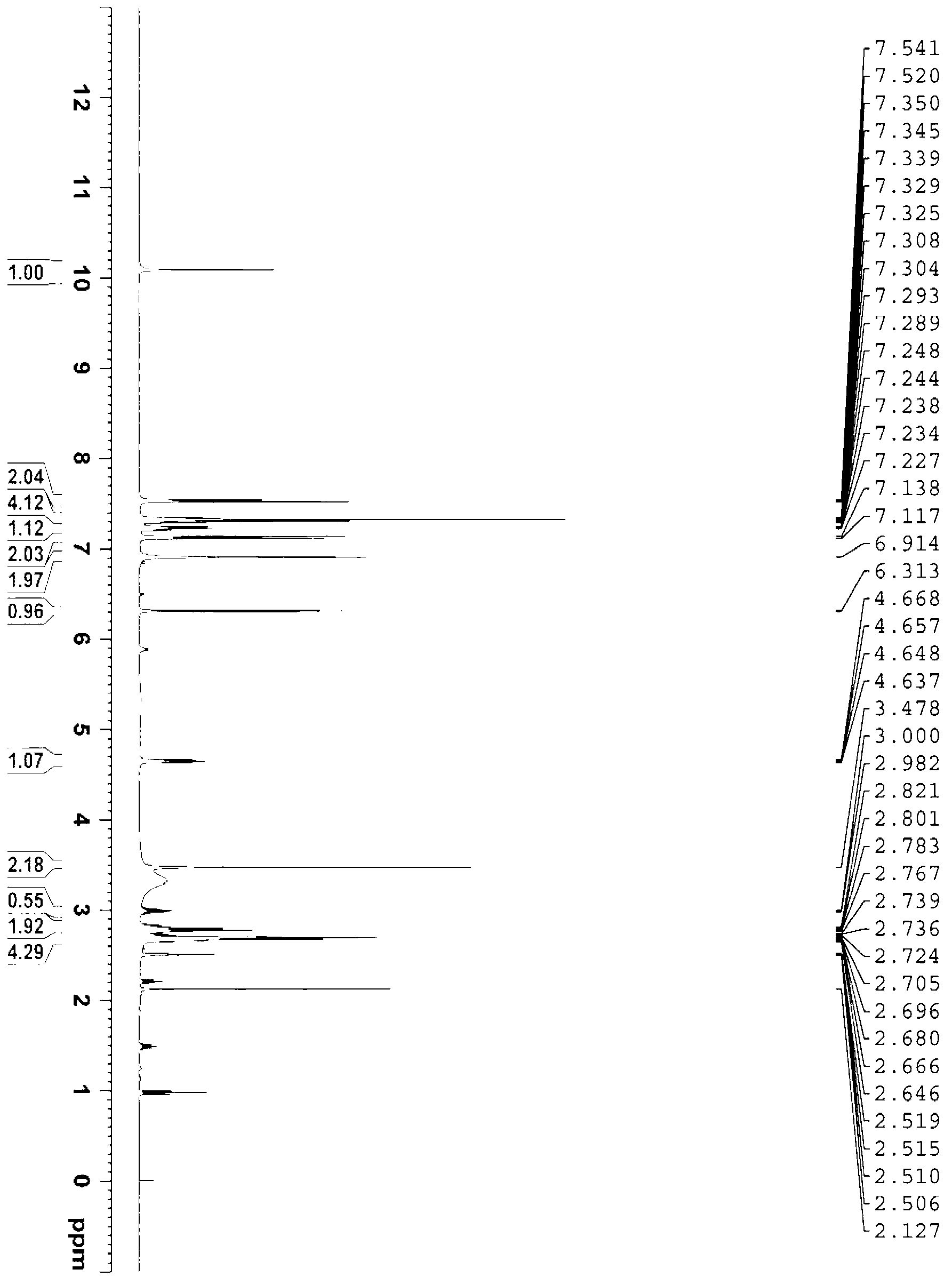
[0065] At 0°C, 1.59g of 2-aminothiazole-4-acetic acid was dissolved in 50mL of tetrahydrofuran to form a reaction solution, and 3.27g of (Boc) 2 O was added dropwise to the reaction solution. The reaction solution was raised to room temperature and stirred for 12 hours to make 2-aminothiazole-4-acetic acid and (Boc) 2 O response. After the completion of the reaction detected by TLC, the reaction solution was concentrated to obtain a crude product, which was recrystallized with ethanol / water to obtain 2.5 g of Mirabegron intermediate product A.
[0066] Add 5.16g of Mirabegron intermediate A to a 100mL three-necked flask and dissolve in 20mL of N,N-dimethylformamide, and stir at 20°C until uniformly mixed to form a mixed solution. Add 3.24g of N,N'-carbonyldiimidazole into a conical flask and dissolve in 10mL of N,N-dimethylformamide, fully dissolve at 30°C and cool to 20°C, then add dropwise to the above mixed solution, drop Stir for 1.5 minutes until TLC and HPLC show comp...
Embodiment 2
[0070] Under the condition of 0° C., 3.16 g of 2-aminothiazole-4-acetic acid was dissolved in 100 mL of methanol to form a reaction liquid, and 3.375 g of benzyloxycarbonyl amino protecting agent was added dropwise into the reaction liquid while stirring. The reaction solution was raised to room temperature and stirred for 12 hours to react 2-aminothiazole-4-acetic acid with benzyloxycarbonyl amino protecting agent. After the completion of the reaction detected by TLC, the reaction solution was concentrated to obtain a crude product, which was recrystallized with ethanol / water to obtain 5.67 g of Mirabegron intermediate product A.
[0071] Add 5.67g of Mirabegron intermediate A to a 100mL three-necked flask and dissolve in 20mL of dichloromethane, and stir at 22°C until uniformly mixed to form a mixed solution. Add 2.97g of N,N'-carbonyldiimidazole into a conical flask and dissolve in 10mL of dichloromethane, fully dissolve at 30°C and cool to 22°C, then add dropwise to the ab...
Embodiment 3
[0075] Under the condition of 0° C., 2.37 g of 2-aminothiazole-4-acetic acid was dissolved in 80 mL of tert-butanol to form a reaction liquid, and 5.49 g of trityl amino protecting agent was added dropwise into the reaction liquid while stirring. The reaction solution was raised to room temperature and stirred for 12 hours to react 2-aminothiazole-4-acetic acid with trityl amino protecting agent. After the reaction was detected by TLC, the reaction solution was concentrated to obtain a crude product, which was recrystallized with ethanol / water to obtain 8.9 g of mirabegron intermediate product A.
[0076] Add 8.9g of Mirabegron intermediate A to a 100mL three-neck flask and dissolve in 20mL of acetonitrile, and stir at 25°C until uniformly mixed to form a mixed solution. Add 5.5g of N,N'-diisopropylcarbodiimide into a conical flask and dissolve it in 10mL of acetonitrile, fully dissolve at 30°C and cool to 25°C, then add dropwise to the above mixed solution, after the drop Stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com