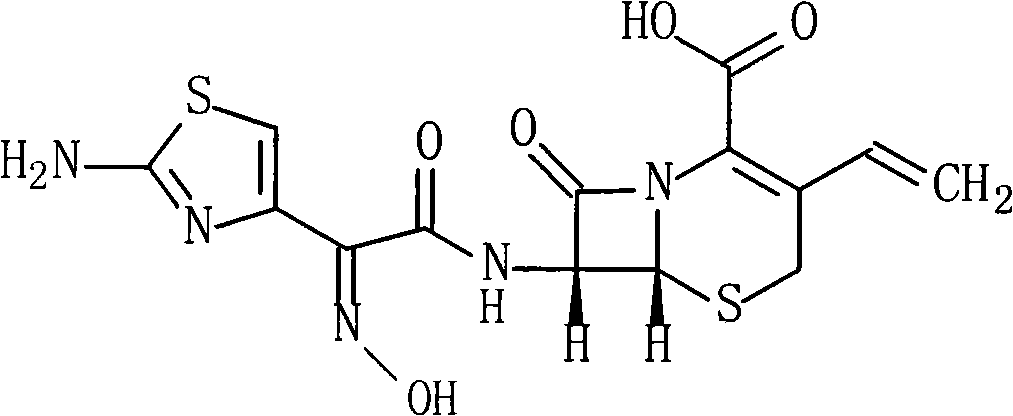
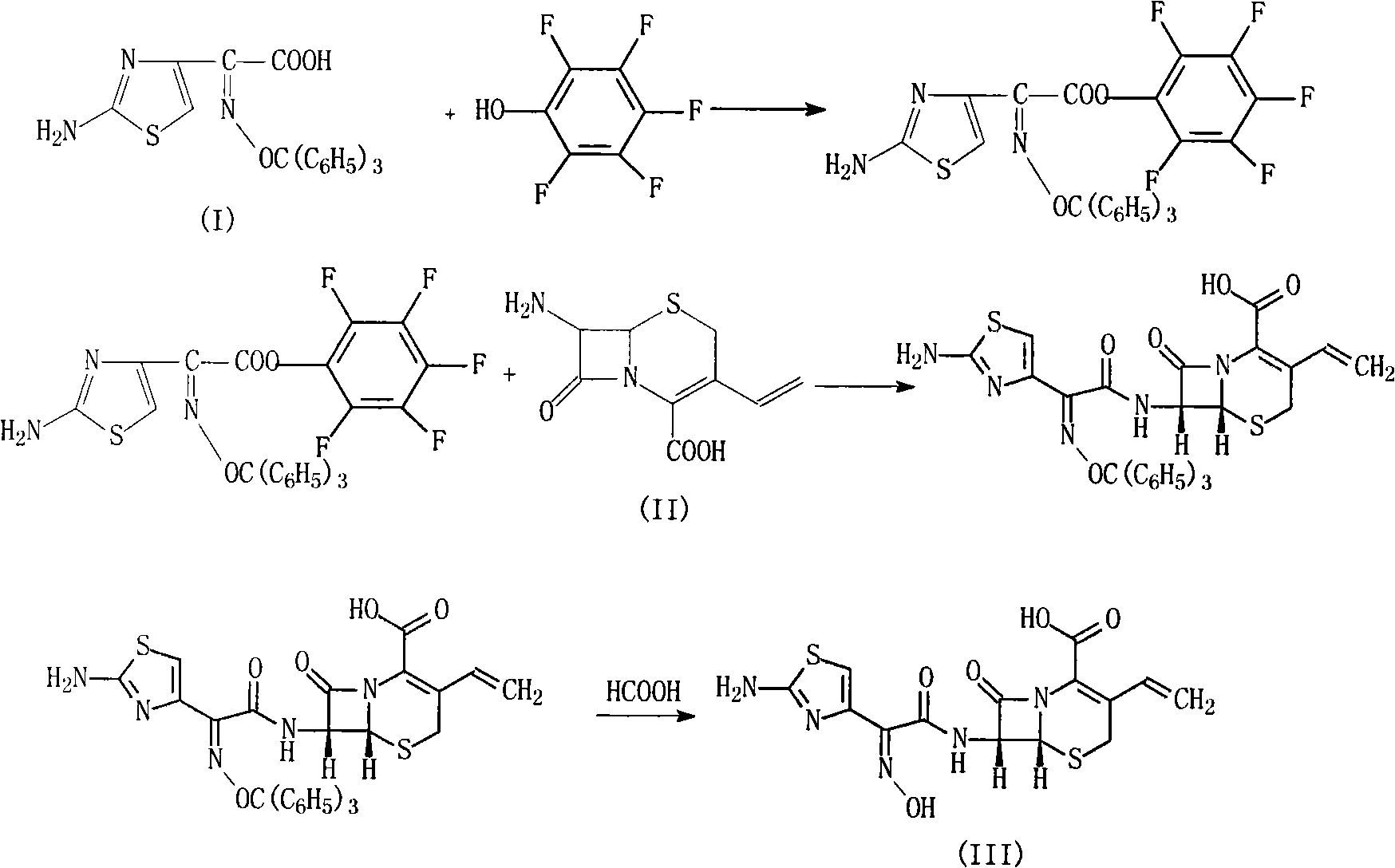
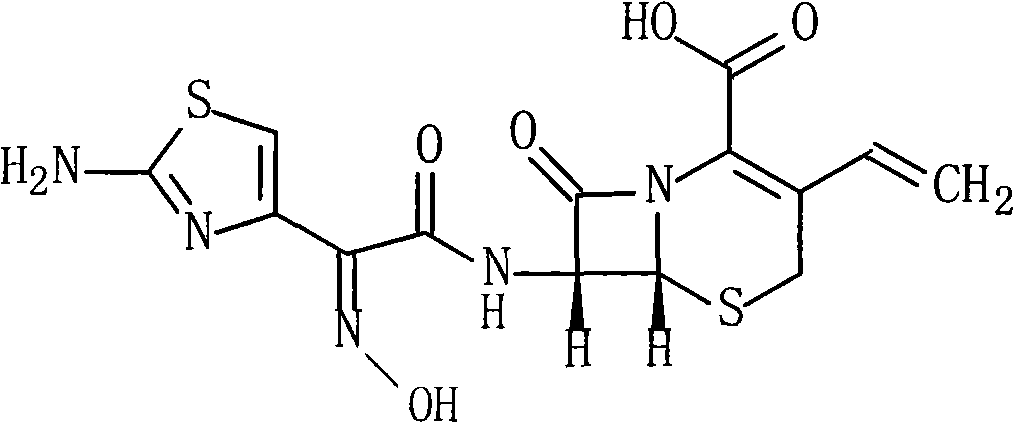
Cefdinir compound and new preparation method thereof
A technology of cefdinir and its compounds, which is applied in the field of cefdinir compounds and its new preparation method, and can solve the problems of large differences in pentafluorophenol structure, complicated operation, and low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 Cefdinir
[0029] (1) 215g (0.5mol) (Z)-2-(2-aminothiazol-4-yl)-2-trityloxyiminoacetic acid and 80ml of triethylamine are added to 350ml of DMF, and the reaction Thing was cooled to 10 ℃, added 93g (0.5mol) pentafluorophenol, stirred reaction at this temperature for 1 hour, then added 113g (0.5mol) 7-AVCA and 140ml triethylamine, stirred vigorously at 10 ℃ for 0.5 hour, then Add 4L of water, adjust the pH of the reaction to 6.5 with 2mol / L hydrochloric acid, stir at room temperature for 1 hour, a solid precipitates, filters, washes with water, and vacuum-dries at 40°C to obtain 287g of the product, yield: 90%.
[0030] (2) 200g (0.31mol) of the above product and 600ml of 99% formic acid were mixed and refluxed for 5 hours, cooled to room temperature, 600ml of water, 300ml of tetrahydrofuran and 600ml of ethyl acetate were added, and the pH was adjusted with 10% aqueous sodium bicarbonate solution to 6.8, Fractionally distill off the water ...
Embodiment 2
[0030] (2) 200g (0.31mol) of the above product and 600ml of 99% formic acid were mixed and refluxed for 5 hours, cooled to room temperature, 600ml of water, 300ml of tetrahydrofuran and 600ml of ethyl acetate were added, and the pH was adjusted with 10% aqueous sodium bicarbonate solution to 6.8, Fractionally distill off the water phase, then wash with 600ml of ethyl acetate, separate the layers, adjust the pH of the water phase to 2.6 with 2mol / L hydrochloric acid, precipitate a solid, stir in an ice bath for 1.5 hours, filter, wash with 200ml of water, at 40 ℃ vacuum drying to obtain 114 g of the product, yield: 92%, purity 99.9%. The synthesis of embodiment 2 Cefdinir
[0031] (1) 215g (0.5mol) (Z)-2-(2-aminothiazol-4-yl)-2-trityloxyiminoacetic acid and 80ml of triethylamine are added to 350ml of DMF, and the reaction Thing was cooled to 5 ℃, added 93g (0.5mol) pentafluorophenol, stirred reaction at this temperature for 1 hour, then added 113g (0.5mol) 7-AVCA and 140ml tri...
Embodiment 3
[0033] The synthesis of embodiment 3 Cefdinir
[0034] (1) 430g (1mol) (Z)-2-(2-aminothiazol-4-yl)-2-trityloxyiminoacetic acid and 150ml of triethylamine are added to 700ml of DMF, and the reactant Cool to 8°C, add 186g (1mol) pentafluorophenol, stir the reaction at this temperature for 1 hour, then add 226g (1mol) 7-AVCA and 280ml triethylamine, stir vigorously at 8°C for 0.5 hour, then add 8L water , adjusted the reaction pH to 6.5 with 5mol / L hydrochloric acid, stirred at room temperature for 1.5 hours, precipitated solid, filtered, washed with water, and dried in vacuum at 45°C to obtain 579g of product, yield: 90.8%.
[0035] (2) 318.8g (0.5mol) of the above product and 1000ml of 99% formic acid were mixed and refluxed for 5 hours, cooled to room temperature, 1000ml of water, 500ml of tetrahydrofuran and 1000ml of ethyl acetate were added, and the pH was adjusted with 10% aqueous sodium bicarbonate to 6.7, the fractional distillation of the aqueous phase was then washed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com