New preparation method of Cefdinir
A technology of cefdinir and solvent, which is applied to the preparation of cefdinir and the preparation field of cefdinir, and can solve the problems of reduced purity, difficult storage, reduced content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
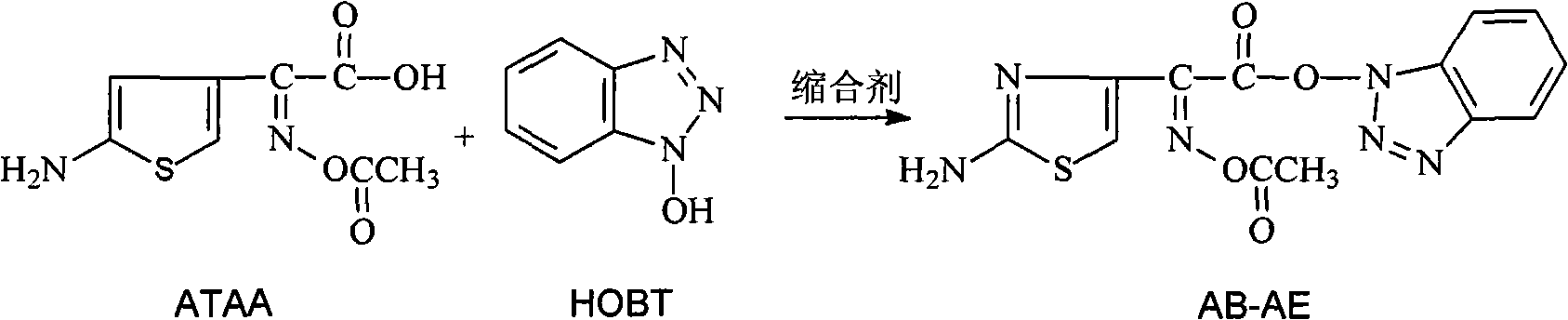
[0049] Add 20g (87.3mmol) (Z)-2-(2-aminothiazol-4-yl)-2-acetoxyiminoacetic acid to a 500ml three-necked sesame cake equipped with mechanical stirring, a constant pressure dropping funnel, and a thermometer (ATAA), 13g (96.0mmol) anhydrous 1-hydroxybenzotriazole (HOBT), 100ml dimethylformamide (DMF), stir at 15--20°C to make it completely dissolved, add dropwise 23.4g (113.5 mmol) dicyclohexylcarbodiimide (DCC), after adding, stir and react at 15--20°C for 30min, filter out the by-product N,N'-dicyclohexylurea, wash with 20ml DMF, add 180ml water to the filtrate, and precipitate A large number of light yellow solids were filtered, and the filter cake was washed with 20ml of DMF solution (volume ratio DMF: water = 1:2), and dried under vacuum at room temperature to obtain 27.8g of 1-[(Z)-2-(2-amino-4-thiazolyl )-2-(acetoxyimino)acetoxy]benzotriazole (AB-AE), yield 92%, purity 98.5%.
[0050] AB-AE 1 HNMR (DMSO-d6):
[0051] &=2.088ppm (s, 3H, COCH3), &=7.431ppm (s, 2H, NH2), ...
Embodiment 2-4
[0054] Embodiment 2-4 is by embodiment 1 method, and the molar ratio of raw and auxiliary materials is also with embodiment 1:
[0055] Example
Embodiment 5
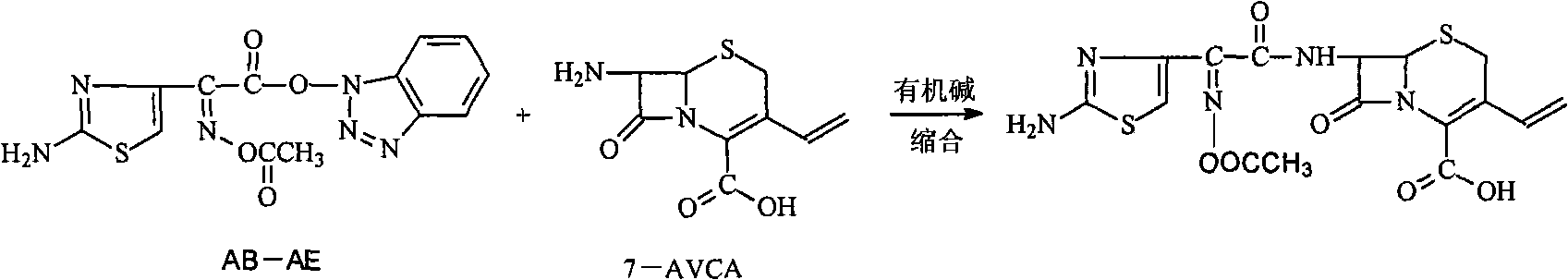
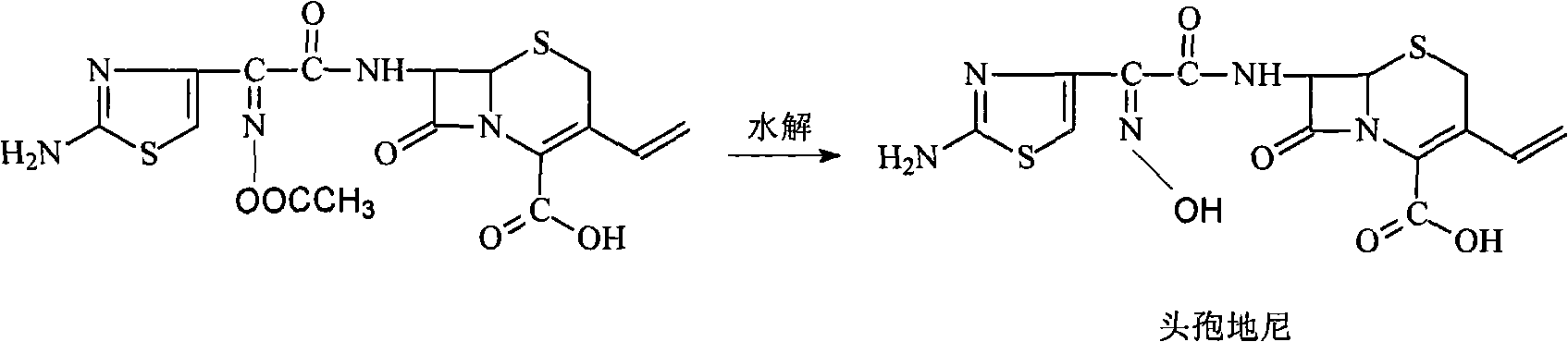
[0057] In the 500ml three-necked sesame cake equipped with mechanical stirring and thermometer, add 16g (70.7mmol) 7-amino-3-vinyl-3-cephalosporin-4-carboxylic acid (7-AVCA), 240ml acetone, 24ml water, Add 9.3g (91.9mmol) triethylamine at 5--10°C to dissolve it completely, then add 28.2g (81.3mmol) AB-AE, react at room temperature until clarification takes about 60min, add 240ml into a 1000ml separating funnel Water, 160ml of dichloromethane to separate layers, transfer the water phase to a 500ml three-necked flask, add saturated sodium carbonate solution dropwise at 5--10°C, control the pH to 8.0-8.5, complete hydrolysis in 45min, and use 5mol / L The sulfuric acid solution was slowly adjusted to pH=3.0. Filter and wash twice with 20ml of water respectively. Dry under vacuum at room temperature. Obtained 25.2 g of light yellow cefdinir, compared with the standard substance, the result was consistent, it was cefdinir, the yield was 90.1%, the purity measured by HPLC: 99.4%, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com