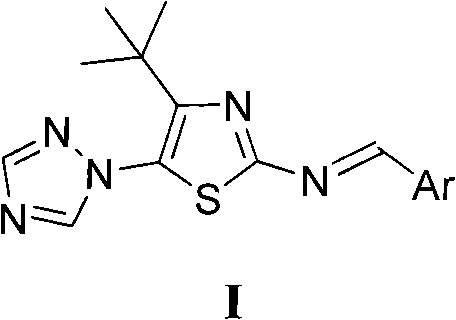
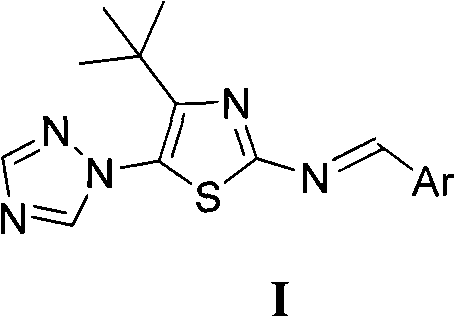
4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole and preparation method and application thereof
A technology of benziminothiazole and aminothiazole, which is applied in the field of new compounds and their preparation, and can solve the problems of no research reports on preparation methods and bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
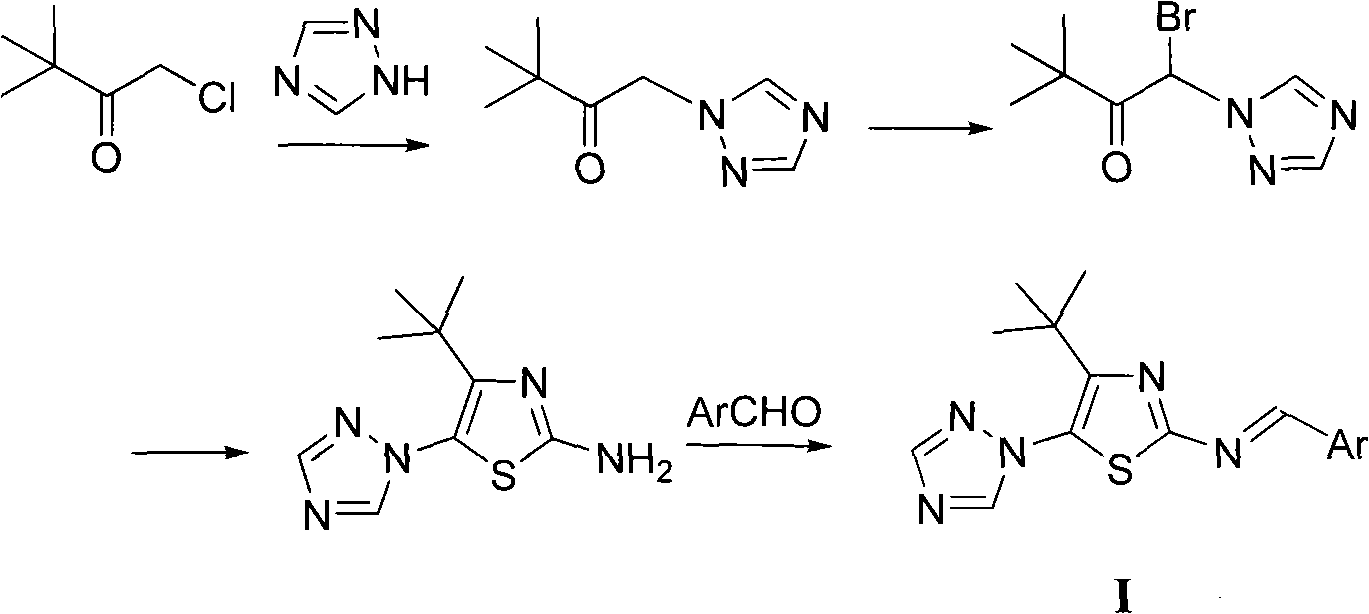
[0019] Example 1 Preparation of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-(2-hydroxybenzylimino)thiazole (Ia)
[0020]
[0021] (1) Preparation of 3,3-dimethyl-1-(1,2,4-triazol-1-yl)-2-butanone
[0022]
[0023] Dissolve 0.05mol 3,3-dimethyl-1-chloro-2-butanone in 60mL ethyl acetate, add 0.05mmol 1,2,4-triazole, add potassium carbonate, reflux for 4.0h, post-treatment to obtain 3 , 3-Dimethyl-1-(1,2,4-triazol-1-yl)-2-butanone, the yield is 80.6%.
[0024] (2) Preparation of 3,3-dimethyl-1-(1,2,4-triazol-1-yl)-1-bromo-2-butanone
[0025]
[0026] 0.03mol 3,3-dimethyl-1-(1,2,4-triazol-1-yl)-2-butanone is dissolved in 45mL acetic acid, 0.03mol sodium acetate is added, 0.03mol bromine is added dropwise, at room temperature After stirring for 6.0 hours, 3,3-dimethyl-1-(1,2,4-triazol-1-yl)-1-bromo-2-butanone was obtained by post-treatment, with a yield of 70.5%.
[0027] (3) Preparation of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-aminothiazole
[0028]
[0029] 0.01mol 3,3-dimethyl-1-(...
Embodiment 2
[0032] Example 2 Preparation of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-(2-hydroxy-3-bromobenzylimino)thiazole (Ib)
[0033]
[0034] (1) Preparation of 2-hydroxy-5-bromobenzaldehyde
[0035]
[0036] 7.5g (0.06mol) 2-hydroxybenzaldehyde is dissolved in 45mL ethanol, and 9.8g Br is added dropwise at 10~15℃ 2 (0.05mol) 45mL CCl 4 The solution was stirred for 0.5h and placed at room temperature for 1h. The precipitate was separated out, filtered with suction, and washed with 95% ethanol until the solution was colorless to obtain white needle-like crystals of 2-hydroxy-5-bromobenzaldehyde. The yield was 73%, and the melting point was 104-106°C.
[0037] (2) Preparation of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-(2-hydroxy-3-bromobenzylimino)thiazole (Ib)
[0038] Dissolve 1mmol 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-aminothiazole in 20mL benzene, add 1mmol 2-hydroxy-5-bromobenzaldehyde, reflux for 1.5h, 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-(2-hydroxy-5-bromobenzylimino)thiazole was o...
Embodiment 3
[0039] Example 3 Preparation of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-(2-hydroxy-3,5-dibromobenzylimino)thiazole (Ic)
[0040]
[0041] (1) Preparation of 2-hydroxy-3,5-dibromobenzaldehyde
[0042]
[0043] Add 25.0g of 40% hydrobromic acid aqueous solution to the flask containing 40mL of glacial acetic acid and 7.5g (0.06mol) of 2-hydroxybenzaldehyde, stir at 30~40℃, slowly add NaClO dropwise 3 The solution reacted for 1.5h, producing a milky white precipitate. Add 30mL of absolute ethanol, warm it to dissolve completely, cool to precipitate white needle crystals, filter with suction, wash with a small amount of ethanol, and dry to obtain 2-hydroxy-3,5-dibromobenzaldehyde, yield 75.2%, melting point 83~85 ℃.
[0044] (2) Preparation of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-(2-hydroxy-3,5-dibromobenzylimino)thiazole (Ic)
[0045] Dissolve 1mmol 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-aminothiazole in 20mL benzene, add 1mmol 2-hydroxy-3,5-dibromobenzaldehyde, and reflux 1.0h to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com