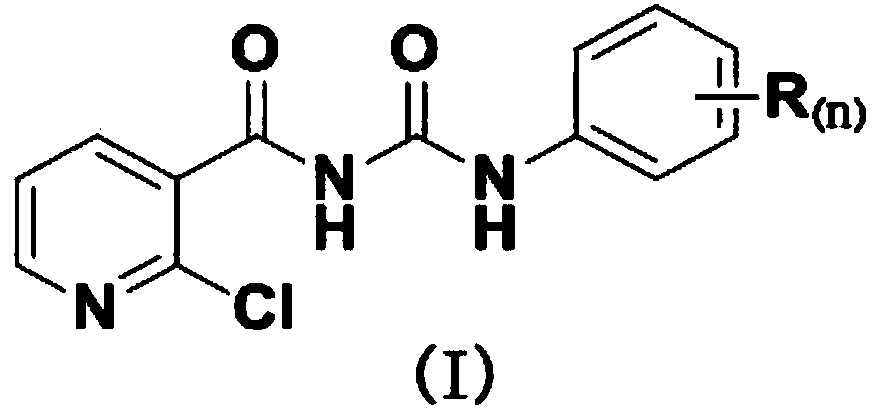
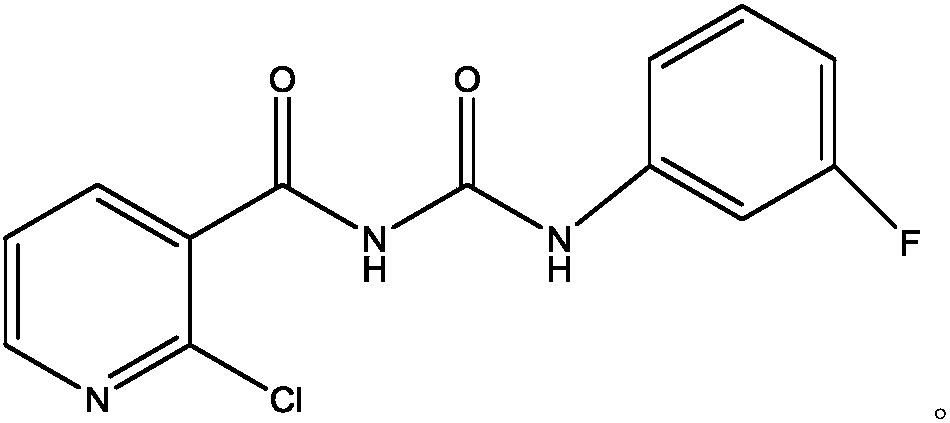
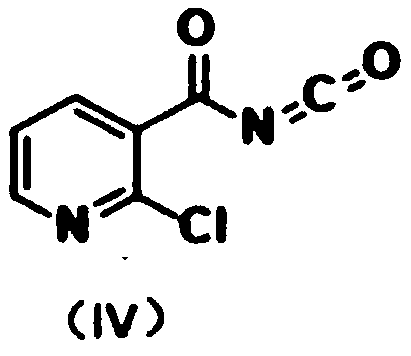
Fluorine-containing nicotinyl urea compounds and preparation method and application thereof
A nicotinyl urea and compound technology, which is applied to fluorine-containing nicotinyl urea compounds and the fields of their preparation and application, can solve the problems of low atom economy, cumbersome separation process, limited range of reaction substrates and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1, The preparation of 2-chloro-N-(4-fluorophenylcarbamoyl)nicotinamide compounds, the following steps are carried out in sequence:
[0047] 1) Add 2-chloronicotinic acid (3.4g, 22mmol) and thionyl chloride (10mL) into a 100mL flask, heat and stir under reflux for 3h, at this time the reaction solution changes from turbid to clear, and continue to reflux for 30min;
[0048] Afterwards, the reaction solution was subjected to rotary evaporation (the rotary evaporation temperature was 50° C.) to remove excess thionyl chloride to obtain a yellow transparent liquid, and the yellow crystal precipitated after the yellow transparent liquid was cooled to room temperature was 2-chloronicotinoyl chloride;
[0049]2) Dilute all the 2-chloronicotinoyl chloride obtained in step 1) with 8mL of dichloromethane to prepare a 2-chloronicotinyl chloride solution for later use; slowly drop the above-prepared 2-chloronicotinyl chloride solution under ice bath conditions Add in 25% (ma...
Embodiment 2
[0056] Change the substituted aniline in step 4) of Example 1 from 4-fluoroaniline to 3,4-difluoroaniline, and the dosage remains unchanged at 1.1 mmol; the rest is the same as in Example 1;
[0057] The final reaction to obtain the target product A2;
[0058] (A2) White solid, yield: 57.6%, melting point: 218-221°C. 1 H NMR (CDCl 3 ,500MHz),δ:7.09-7.18(m,2H,Ph),7.45-7.48(m,1H,Py),7.55-7.59(m,1H,Ph),8.10-8.22(m,1H,Py), 8.62-8.64(m,1H,Py),9.47(s,1H,NH),10.52(s,1H,NH);HRMS(ESI)for C 13 h 8 CIF 2 N 3 o 2 m / z: Calculated, 312.0346, Found, 312.0364[M+H] + .
[0059] The structural formula of A2 is:
[0060]
Embodiment 3
[0062] Change the substituted aniline in step 4) of Example 1 from 4-fluoroaniline to 4-trifluoromethylaniline, and the amount remains unchanged at 1.1 mmol; the rest is the same as in Example 1;
[0063] Final reaction obtains target product A3;
[0064] (A3) White solid, yield: 57.9%, melting point: 213-215°C. 1 H NMR (CDCl 3 ,500MHz), δ:7.56-7.59(m,1H,Py),7.71(d,J=6.8Hz,2H,Ph),7.81(d,J=6.8Hz,2H,Ph),8.11-8.13(m ,1H,Py),8.55-8.57(m,1H,Py),10.54(s,1H,NH),11.44(s,1H,NH); HRMS(ESI)forC 14 h 9 CIF 3 N 3 o 2 m / z: Calculated, 344.0408, Found, 344.0444[M+H] + .
[0065] The structural formula of A3 is:
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com