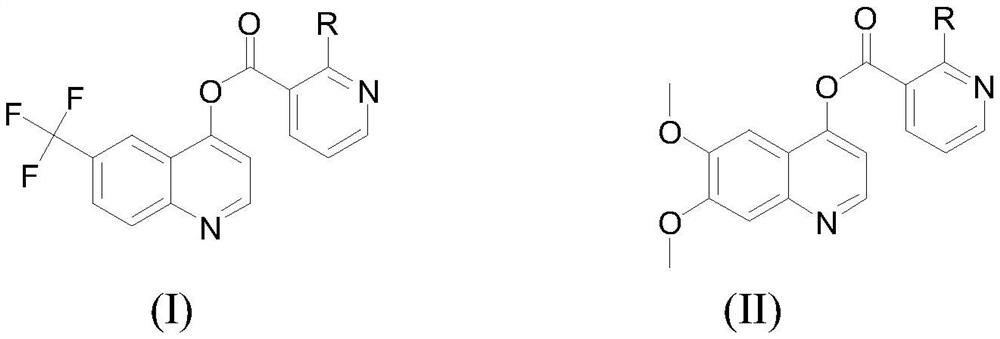
Quinoline 4-hydroxypicolinate compound and application thereof in prevention and treatment of magnaporthe oryzae
A technology of hydroxypicolinic acid and rice blast fungus, applied in application, fungicide, organic chemistry and other directions, can solve the problem of no research and development report on the activity of rice blast fungus, and achieve the effect of improving comprehensive performance and good environmental compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
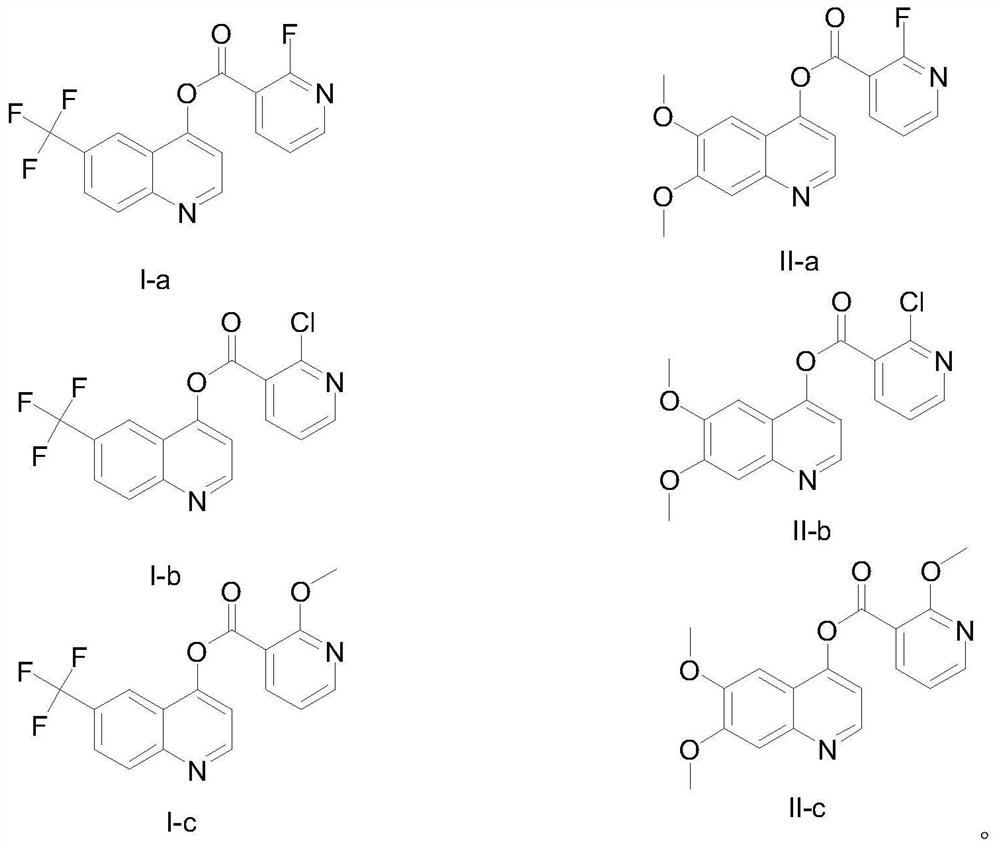
[0030] Preparation of 6-(trifluoromethyl)-quinolin-4-yl(2-fluoropyridine)carboxylate (I-a)
[0031] 2-Fluoropyridine-3-carboxylic acid (1.1 mol) and intermediate 6-trifluoromethyl-4-quinolinol (1.0 mmol) were dissolved in 3 mLofCH 2 Cl 2 The solvent was stirred and cooled down to 0°C, and EDCI (1.2 mmol) and DMAP (1.2 mmol) were added to the above system. The reaction was carried out at 40°C under nitrogen protection for 15h-20h. After the reaction was completed, the reaction solution was washed with saturated brine (2×2mL), and washed with water (2×2mL), dried over sodium sulfate, filtered with suction, and the crude product was obtained by flash column chromatography (petroleum ether / ethyl acetate, v / v=10 :1–1:1) to obtain compound 6-(trifluoromethyl)-quinolin-4-yl(2-fluoropyridine)carboxylate, white powder, yield 51%, 1 H NMR (400MHz, CDCl 3 )δ: 9.08 (d, J = 4.8Hz, 1H), 8.62–8.67 (m, 1H), 8.58 (d, J = 4.4Hz, 1H), 8.45 (s, 1H), 8.29 (d, J 1 =8.8Hz,1H),7.95(dd,J 1 =8.8Hz...
Embodiment 2
[0033] Preparation of 6-(trifluoromethyl)-quinolin-4-yl(2-chloropyridine)carboxylate (I-b)
[0034] White powder, yield 37%, 1 H NMR (400MHz, CDCl 3 )δ: 9.11(d,J=4.8Hz,1H), 8.70(dd,J 1 =4.8Hz,J 2 =2.0Hz,1H),8.51(dd,J 1 =8.0Hz,J 2 =2.0Hz,1H),8.41(s,1H),8.31(d,J 1 =8.8Hz,1H),7.96(dd,J 1 =8.8Hz,J 1 =1.5Hz,1H),7.64(d,J=4.8Hz,1H),7.53(dd,J 1 =8.0Hz,J 2 = 4.8Hz, 1H).
Embodiment 3
[0036] Preparation of 6-(trifluoromethyl)-quinolin-4-yl(2-methoxypyridine)carboxylate (I-c)
[0037] White powder, yield 26%, 1 H NMR (400MHz, CDCl 3 )δ:9.07(d,J=4.6Hz,1H), 8.63(s,1H),8.48(dd,J 1 =4.8Hz,J 2 =1.7Hz,1H),8.44(dd,J 1 =7.5Hz,J 2 1.7 Hz, 1H), 8.28(d, J=8.8Hz, 1H), 7.95(d, J=8.8Hz, 1H), 7.72(d, J=4.9Hz, 1H), 7.11 (dd, J 1 =7.5Hz,J 2 =4.9Hz, 1H), 4.19(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com