Preparation methods of high-purity cefotiam hexetil and dihydrochloride of high-purity cefotiam hexetil
A technology of cefotiam and cefotiam, which is applied in the field of preparation of high-purity cefotiam and its dihydrochloride, can solve problems such as being unsuitable for industrialized large-scale production, unsuitable for industrialized large-scale production, and high in product impurities. problem, to achieve the effect of small impurities, simple and easy preparation method, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 high-purity cefotiam
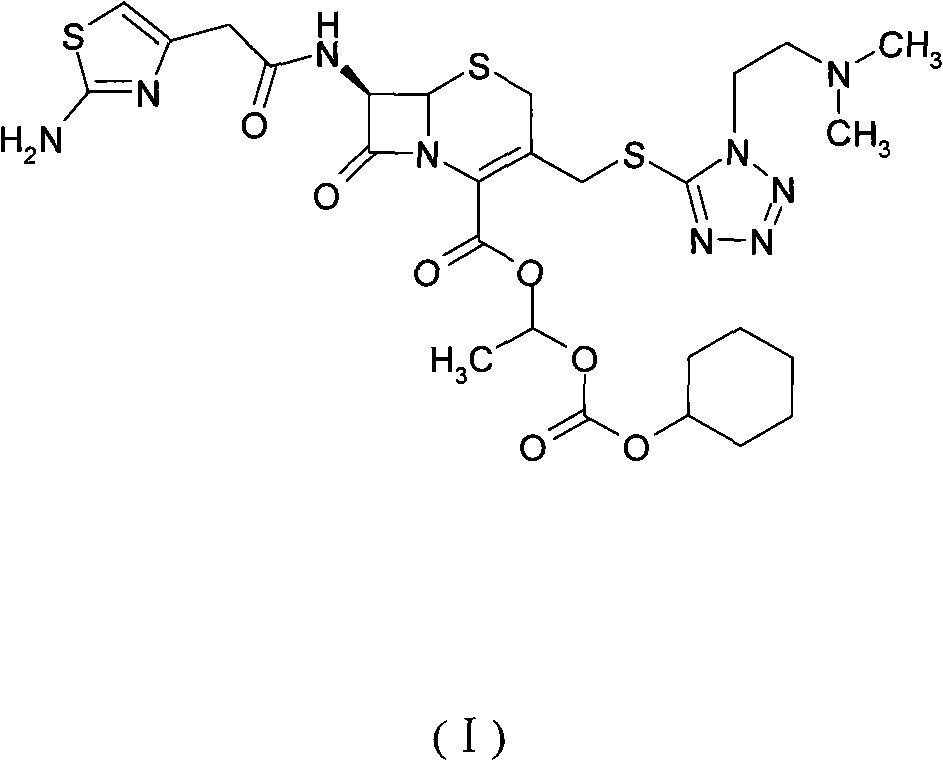
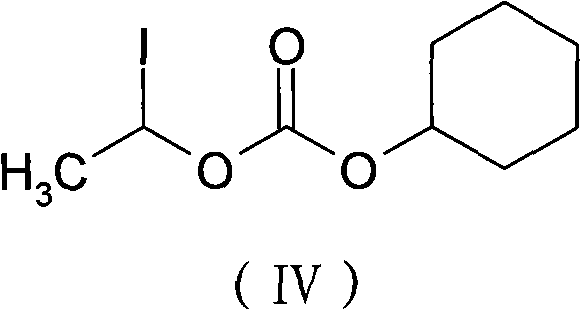
[0039] Add DMA (160ml) to a 500ml reaction flask, add cefotiam potassium (20g, 0.035mol), stir until completely dissolved, cool to -5°C, add anhydrous potassium carbonate (4.84g, 0.035mol), add carbonic acid- 1-iodoethyl cyclohexyl ester (21.14g, 0.07mol), stir until the reaction is complete, add 700ml of ethyl acetate to the reaction solution, add 350ml of purified water, let stand to separate layers, add 0.5mol / L hydrochloric acid solution 140ml of aqueous solution, stirring, standing for stratification, adding 700ml of ethyl acetate (a benign solvent), adjusting the pH value to 6.0 with 2% sodium bicarbonate solution, then standing for stratification, and drying the organic layer with anhydrous magnesium sulfate and activated carbon Remove impurities, filter, concentrate the filtrate, add 74ml of methanol (good solvent) to the residue, add 400ml of isopropyl ether (crystallization solvent), stir and crystallize ...
Embodiment 2
[0042] The preparation of embodiment 2 high-purity cefotiam
[0043] Add DMA (160ml) to a 500ml reaction flask, add cefotiam sodium (20g, 0.035mol), stir until completely dissolved, cool to 0°C, add anhydrous potassium carbonate (7.26g, 0.053mol), add carbonic acid-1 - Iodoethyl cyclohexyl ester (21.14g, 0.07mol), after stirring until the reaction is complete, add 700ml of ethyl acetate to the reaction solution, add 350ml of purified water, let stand to separate layers, add 140ml of 0.5mol / L hydrochloric acid solution Aqueous solution, stirring, static layering, adding 700ml of ethyl acetate, adjusting the pH value to 5.8-6.5 with 2% sodium bicarbonate solution, then static layering, the organic layer was dried with anhydrous magnesium sulfate and activated carbon to remove impurities, Filtrate, concentrate the filtrate, add 74ml of methanol to the residue, add 400ml of isopropyl ether, stir and crystallize at 15-20°C for 2h, filter, wash the filter cake with isopropyl ether, ...
Embodiment 3
[0046] The preparation of embodiment 3 high-purity cefotiam axetil
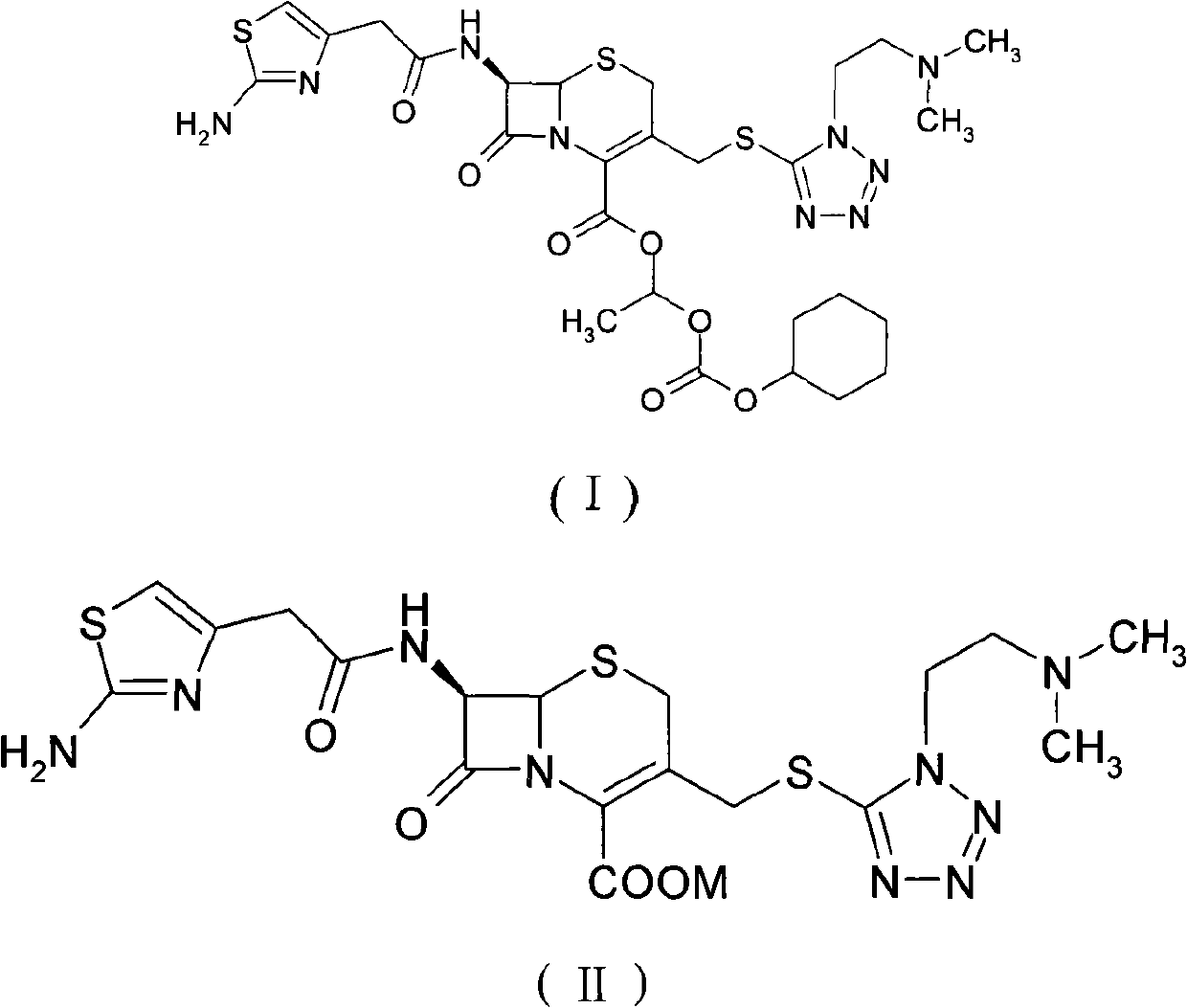
[0047] Add 14 g of the above-prepared cefotiam to the reaction bottle, add 42 ml each of methanol and acetone, dissolve, add 300 ml of isopropyl ether, stir and crystallize at 15-20 ° C for 2 hours, filter to obtain 12 g of white cefotiam, Yield 85.7%.
[0048] Detected by HPLC: purity 99.3%; Δ 2 Isomers: 0.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com