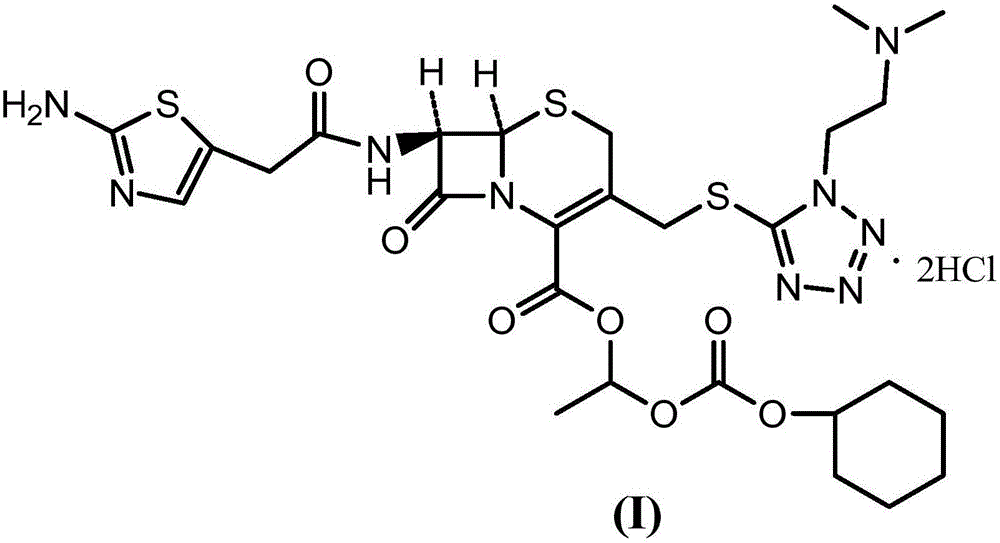
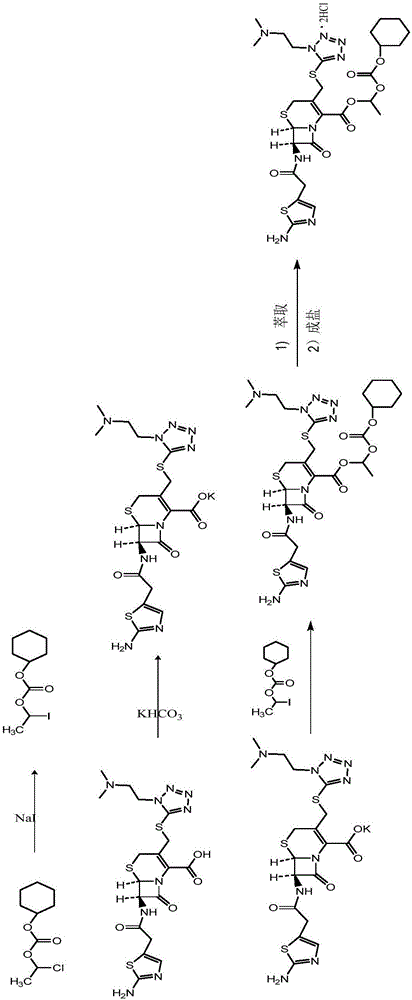
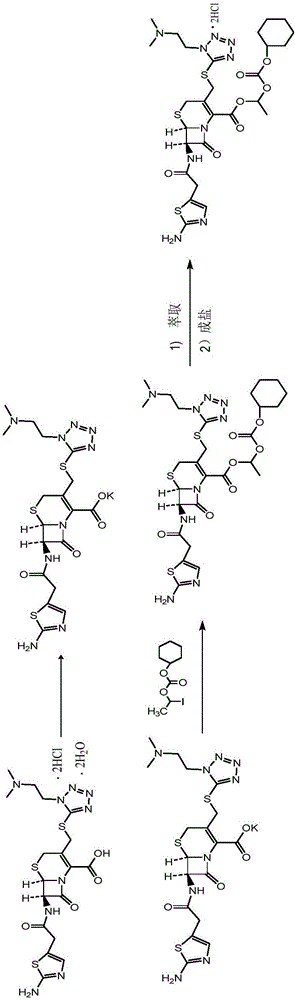
Method for preparing cefotiam hexetil hydrochloride
A technology of cefotiam hydrochloride and cefotiam, which is applied in the field of preparation of cefotiam hydrochloride, can solve the problems of purification of the key intermediate cefotiam free base, poor stability of iodocarbonate, difficulty in obtaining purity of finished products, etc. , to ensure the quality and stability of the process, avoid the deterioration of intermediates, and reduce the effect of reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] A kind of preparation method of cefotiam hydrochloride, concrete steps are as follows:
[0083] Step (1) Potassium salt formation reaction: add 0.50 kg of purified water, 0.47 kg of potassium bicarbonate, and 4.0 kg of butanone into a dry and clean reaction tank. Start stirring, raise the temperature to 20-25°C, then slowly add 1.0Kg of cefotiam hydrochloride (compound II), and keep the temperature of the material at 20-25°C after the addition, and stir for 0.5-1h. After the reaction is completed, control the temperature T≤50°C, pressure≤-0.09mMpa, and evaporate butanone under reduced pressure to obtain the potassium salt of cefotiam (compound IIa). The product in this step is not dried, and the yield is directly used in the next step according to 100% calculation.
[0084] Step (2) Esterification reaction: Add 4.5 kg of N,N-dimethylacetamide to the residue (theoretical yield 0.89 kg) of the potassium salt (compound IIa) of cefotiam in the above step (1), cool to -10 ...
Embodiment 2
[0088] A kind of preparation method of cefotiam hydrochloride, concrete steps are as follows:
[0089] Step (1) Potassium salt formation reaction: add 1.0 kg of purified water, 0.93 kg of potassium acetate, and 8.0 kg of acetone into a dry and clean reaction tank. Start stirring, raise the temperature to 25-30°C, then slowly add 1.0Kg of cefotiam hydrochloride (compound II), after the addition, keep the material temperature at 25-30°C, and stir for 0.5-1h. After the reaction is completed, the temperature is controlled at 20-25° C., the pressure is ≤-0.09 mMpa, and the acetone is evaporated under reduced pressure to obtain the potassium salt of cefotiam (compound IIa). The product in this step is not dried, and the yield is directly used in the next step according to 100% calculation.
[0090] Step (2) Esterification reaction: Add 8.9 kg of N,N-dimethylformamide to the residue (theoretical yield: 0.89 kg) of the potassium salt of cefotiam (compound IIa) in the above step (1), ...
Embodiment 3
[0094] A kind of preparation method of cefotiam hydrochloride, concrete steps are as follows:
[0095] Step (1) Potassium salt formation reaction: add 0.7 kg of purified water, 0.65 kg of potassium bicarbonate, and 6.0 kg of acetone into a dry and clean reaction tank. Start stirring, raise the temperature to 25-30°C, then slowly add 1.0Kg of cefotiam hydrochloride (compound II), after the addition, keep the material temperature at 25-30°C, and stir for 0.5-1h. After the reaction is completed, the temperature is controlled at 25-30° C., the pressure is ≤-0.09 mMpa, and the acetone is evaporated under reduced pressure to obtain the potassium salt of cefotiam (compound IIa). The product in this step is not dried, and the yield is directly used in the next step according to 100% calculation.
[0096]Step (2) Esterification reaction: Add 7.6 kg of N,N-dimethylacetamide to the residue (theoretical yield 0.89 kg) of the potassium salt (compound IIa) of cefotiam in the above step (1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com