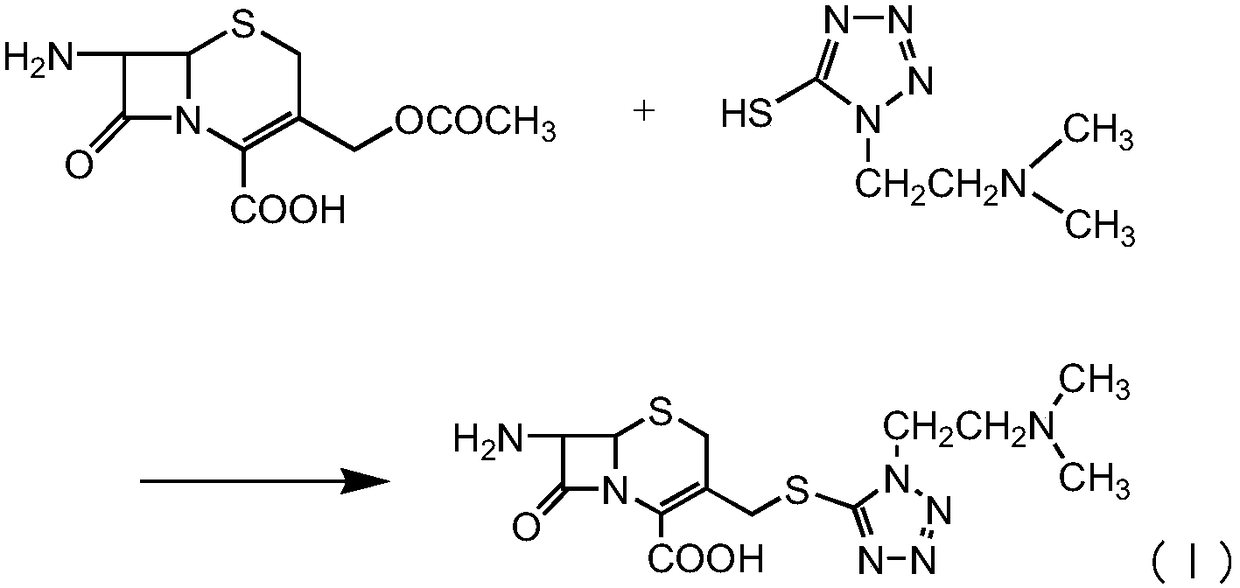
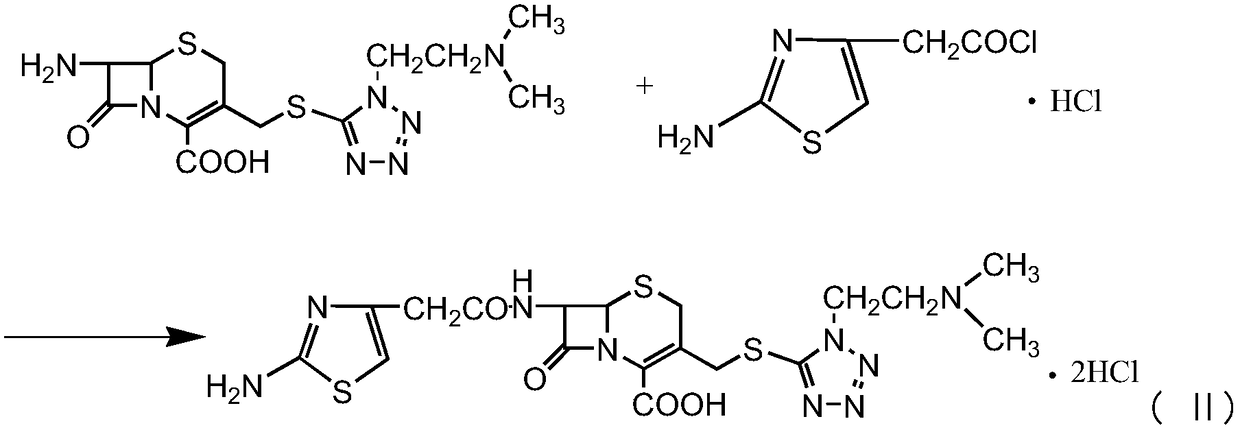
Preparation method for cefotiam hydrochloride
A technology of cefotiam hydrochloride and hydrochloric acid, applied in a new preparation field, can solve problems such as unfavorable production, research and utilization, complicated reaction process, poor reaction degree, etc., achieve less residual impurities, high content, and reduce environmental protection pressure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0035] Example 1-1, Preparation of aminothiazole acetyl chloride hydrochloride (ATC.HCL).
[0036] Add 120 ml of acetonitrile to the reactor to control the temperature below 10 degrees, and add DMF. 50 g of phosphorus oxychloride was added dropwise, and 60 g of aminothiazole acetic acid hydrochloride was added after dropping, and the reaction was timed for 10-20 hr, then the temperature was lowered, and 120 ml of acetonitrile was added. Crystal growth 1.0-2.0hr. Centrifuged aminothiazole acetyl chloride hydrochloride (ATC.HCL), high pressure liquid phase purity 98%
Embodiment 1-2
[0038] Add 100 ml of acetonitrile to the reactor to control the temperature below 10 degrees, and add DMF. Add 60 g of thionyl chloride dropwise, and add 60 g of aminothiazole acetic acid hydrochloride after dropping, time the reaction for 4-6 hours, then lower the temperature, and add 100 ml of acetonitrile. Crystal growth 1.0-2.0hr. Centrifuged aminothiazole acetyl chloride hydrochloride (ATC.HCL), high pressure liquid phase purity 97.5%
Embodiment 2
[0040] At room temperature, 100 ml of dimethyl carbonate, 40 g of 7-aminocephalosporanic acid, and 15 g of MTZ were added. Add 15g of boron trifluoride-dimethyl carbonate complex and 5g of methanesulfonic acid at the same temperature, control the temperature at 35-40°C and time the reaction for 1-2.0hr, then cool down to below 20°C and add 320ml of pure water for hydrolysis, then add 300ml Carbon tetrachloride was extracted for 10 minutes, and the layers were separated. Then add activated carbon to decolorize for 20min and filter. Control the temperature of the filtrate below 5°C, add triethylamine dropwise to adjust the pH to 7.0-8.0, then add ATC.HCL (Example 1-1), and react at a time below 5°C for 1-2.0 hours. After the reaction is complete, add Add 60ml of concentrated hydrochloric acid, then add activated carbon for decolorization for 20min, filter, add 1400-1500ml of acetone dropwise to the filtrate, add seed crystals to grow the crystal, after the crystallization is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com