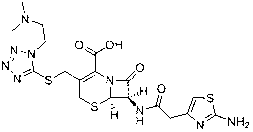
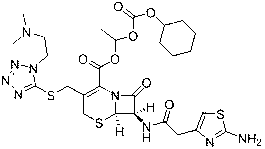
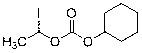
Novel preparation technology of cefotiam hexetil hydrochloride
A cefotiam pivoxil and a preparation process technology, applied in the direction of organic chemistry and the like, can solve problems such as being unsuitable for industrialized large-scale production, poor reproducibility of product qualification, corrosion of equipment and environment, etc., achieving simple and fast post-processing and salt-forming processes, The preparation method is simple and easy to implement, and the damage to the equipment and the environment is reduced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 Preparation of high-purity cefotiam axetil hydrochloride
[0053] The specific steps of the preparation method of the present embodiment cefotamate hydrochloride are as follows:
[0054] 1) Add 300mL of acetonitrile and 38.8g of anhydrous sodium iodide into a 500mL reaction flask, stir to dissolve, add 38.2g of 1-chloroethylcyclohexyl carbonate, replace with nitrogen three times, control the temperature at 55~60°C, and react in the dark for 1h. Concentrate under reduced pressure to dryness after the reaction, add 200mL ethyl acetate and 280mL water to the residue, extract, collect the organic layer, add 160mLNa 2 S 2 o 3 (5%) washing, the organic phase was collected, and the solvent was evaporated to dryness under reduced pressure to obtain 44g brownish-yellow oily matter which was 1-iodoethylcyclohexyl carbonate.
[0055] 2) Add 260mL DMF and 48g cefotiam hydrochloride into a 2000mL reaction flask, stir to dissolve at room temperature, cool down to -5~0...
Embodiment 2
[0057] Example 2 Preparation of high-purity cefotiam axetil hydrochloride
[0058] The specific steps of the preparation method of cefotamate hydrochloride in the present embodiment are as follows:
[0059] 1) Add 600mL of acetone and 78g of anhydrous sodium iodide into a 1000mL reaction flask, stir to dissolve, add 76.5g of 1-chloroethylcyclohexyl carbonate, replace with nitrogen three times, control the temperature at 55~60°C, and react in the dark for 1h. Concentrate under reduced pressure to dryness after the reaction, add 400mL ethyl acetate and 550mL water to the residue, extract, collect the organic layer, add 350mLNa 2 S 2 o 3 (5%) was washed, the organic phase was collected, and the solvent was evaporated to dryness under reduced pressure to obtain 84 g of 1-iodoethylcyclohexyl carbonate of brown oil.
[0060] 2) Add 540mL DMF and 100g cefotiam hydrochloride into a 5000mL reaction flask, stir to dissolve at room temperature, cool down to -5~0°C, add 37.2g micronize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com