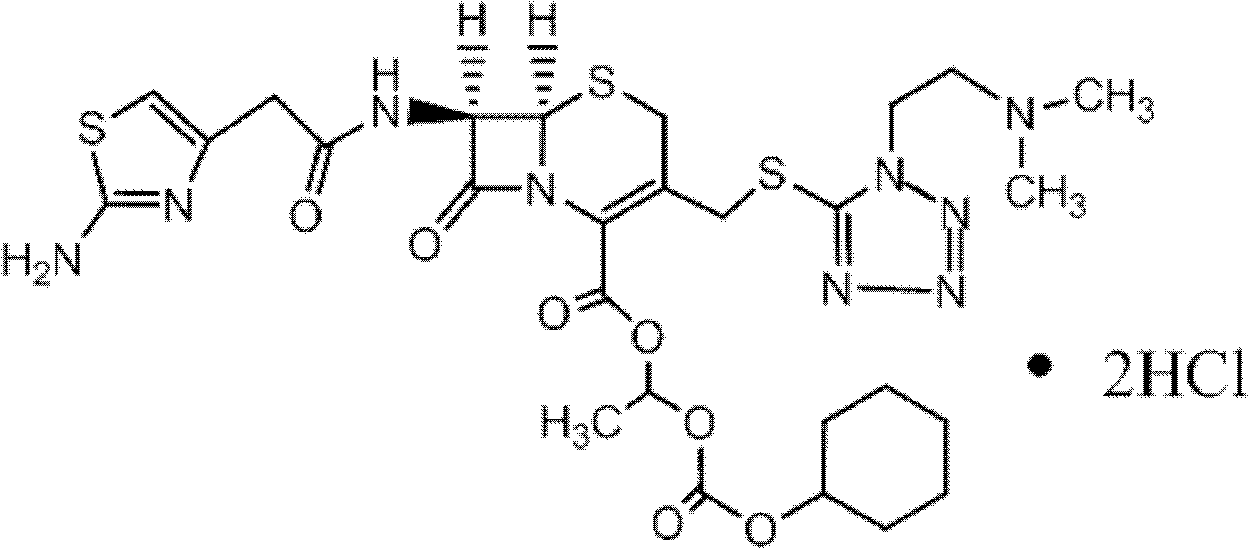
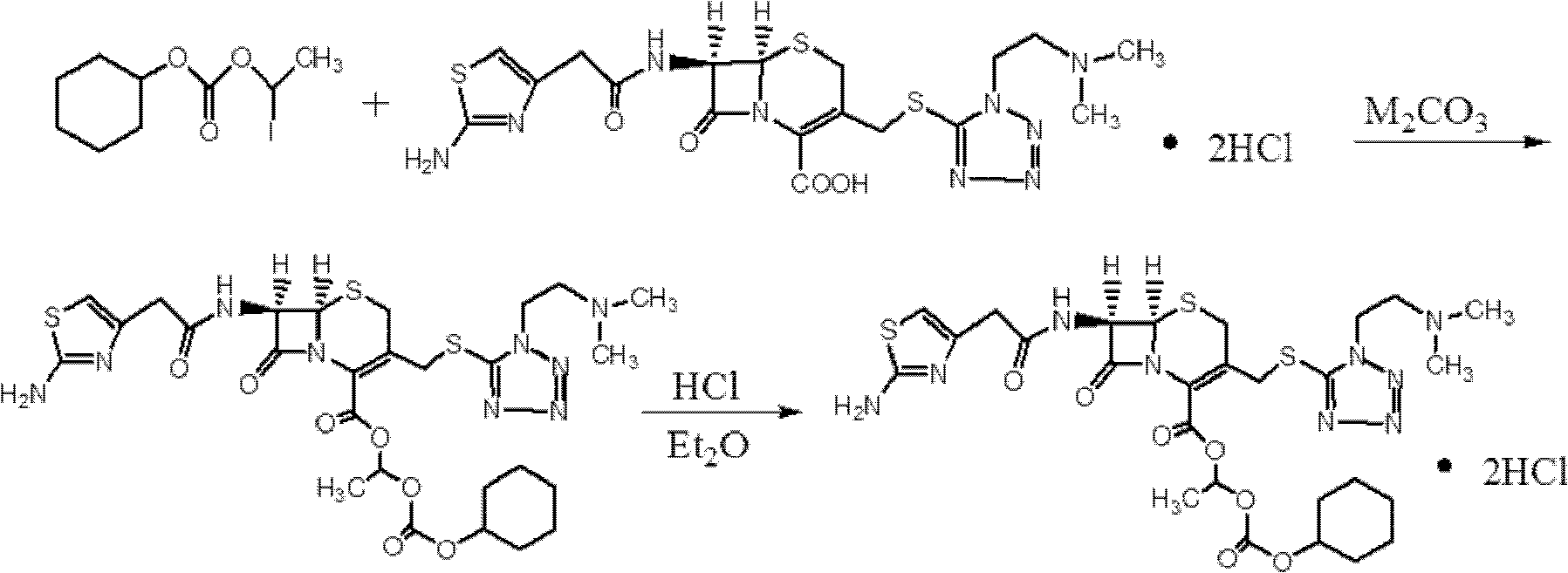
Preparation method of cefotiam hexetil hydrochloride
A technology for cefotiam hydrochloride and cefotiam hydrochloride, which is applied in the field of antibiotic preparation, can solve the problems of expensive raw materials, high isomer ratio, complicated purification means, etc., and achieves the advantages of simplifying the purification method and avoiding the use of chromatographic columns. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1) Add 120ml of N,N-dimethylformamide into a 250ml dry three-neck flask, cool down and stir. When the temperature of the solution in the bottle dropped to 0° C., 12 g (0.020 mol) of cefotiam hydrochloride was added and stirred to completely dissolve it.
[0059] 2) Lower the temperature. When the temperature of the solution in the bottle drops to -3°C, add 3.0 g (0.021 mol) of anhydrous potassium carbonate for the first time, and stir for 1 h. After 1 hour, the temperature was lowered. When the temperature in the bottle dropped to -5°C, 3.2 g (0.023 mol) of anhydrous potassium carbonate was added for the second time and stirred for 2 hours.
[0060] 3) Add 16.0 g (0.050 mol) of 1-iodoethylcyclohexyl carbonate, which has been cooled in an ice bath in advance, to the reaction solution. At this time, ensure that the temperature of the solution in the three-necked flask is -4 ° C ~ -6 ° C, and the reaction 8 minutes.
[0061] 4) after the reaction is finished, the reactio...
Embodiment 2
[0067] 1) Add 120ml of N,N-dimethylformamide into a 250ml dry three-neck flask, cool down and stir. When the temperature of the solution in the bottle dropped to 0° C., 12 g (0.020 mol) of cefotiam hydrochloride was added and stirred to completely dissolve it.
[0068] 2) Lower the temperature. When the temperature in the bottle drops to -5°C, add 6.2 g (0.044 mol) of anhydrous potassium carbonate at one time, and after stirring for 2.5 hours, add the 1-iodoethylcyclohexyl carbonate that has been cooled in a low-temperature bath to 16.5 g (0.055 mol) of the ester was added to the reaction solution, and at this time, the temperature of the solution in the three-neck flask was kept at -4°C-6°C, and the esterification reaction was carried out for 10 minutes.
[0069] 3) after the reaction finishes, the reaction solution is poured into the ice mixed solution of 300ml5% sodium chloride and 300ml ethyl acetate, the organic phase ethyl acetate solution is separated, the water phase i...
Embodiment 3
[0075] 1) Add 120ml of N,N-dimethylformamide into a 250ml dry three-neck flask, cool down and stir. When the temperature of the solution in the bottle dropped to 0° C., 12 g (0.020 mol) of cefotiam hydrochloride was added and stirred to completely dissolve it.
[0076] 2) Lower the temperature. When the temperature of the solution in the bottle drops to -3°C, add 3.5 g (0.025 mol) of anhydrous potassium carbonate for the first time, and stir for 1 h. After 1 hour, lower the temperature. When the temperature in the bottle drops to -5°C, add 3.2 g (0.023 mol) of anhydrous potassium carbonate for the second time. After stirring for 2 hours, add 1-iodoethylcyclohexylcarbonic 16.0 g (0.050 mol) of the ester was added to the reaction solution (the temperature of the solution in the three-neck flask was kept at -4°C-6°C), and the esterification reaction was carried out for 8 minutes.
[0077] 3) after the reaction is finished, the reaction solution is poured into the ice mixed solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com