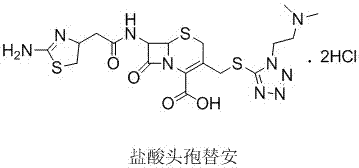
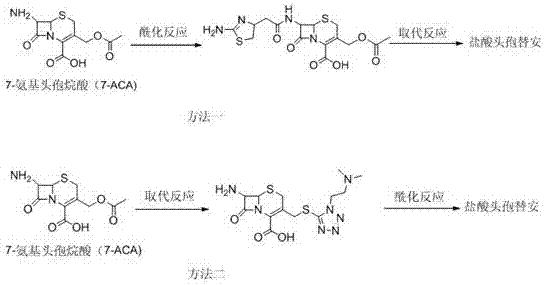
New cefotiam hydrochloride synthesis method and applications of cefotiam hydrochloride in sterile powder injection
A technology of cefotiam hydrochloride and hydrochloric acid, which is applied in the field of synthesis of cefotiam hydrochloride, can solve the problems of infeasibility, excessively increasing the consumption of 2-mercaptobenzimidazole compounds, and low yield, so as to avoid long reaction steps, Environmental friendliness and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
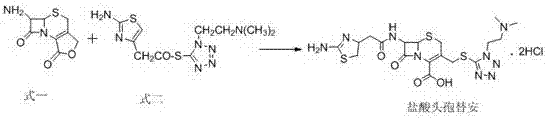
[0019] Reference example 1 preparation of formula one compound
[0020] Add 20g (73.5mmol) of 7-aminocephalosporanic acid and 7.5g (188mmol) of sodium hydroxide into 100mL of ethanol aqueous solution, react at -15°C for 2h, adjust the pH value to 1-2 with hydrochloric acid, keep the reaction for 1h, and separate to obtain Formula 1 compound 13g.
Embodiment 1
[0021] The preparation of embodiment 1 cefotiam hydrochloride
[0022] Add 10 g (47 mmol) of the compound of formula 1, 22 g (70 mmol) of the compound of formula 2, and 10 mL of 1 mol / L hydrochloric acid aqueous solution into 100 mL of acetonitrile, and react at 45°C for 3 h. Evaporate most of the acetonitrile under reduced pressure, add 50mL of water, 10mL of concentrated hydrochloric acid, stir at room temperature for 1h, filter with suction, cool the filtrate to 0-5°C, add acetone dropwise until a large amount of turbidity appears, keep stirring for 1h, continue to add 300mL of acetone dropwise, keep warm Stir for 1 h, filter and dry with suction to obtain 25 g of white cefotiam hydrochloride crystalline powder, with a molar yield of 88%, an HPLC content of 98.30%, and a maximum of 0.45% simple impurities.
Embodiment 2
[0023] The preparation of embodiment 2 cefotiam hydrochloride
[0024] Add 20 g (94 mmol) of the compound of formula 1, 38 g (122 mmol) of the compound of formula 2, and 19 mL of 1 mol / L hydrochloric acid aqueous solution into 200 mL of acetonitrile, and react at 30°C for 6 h. Evaporate most of the acetonitrile under reduced pressure, add 100mL of water, 20mL of concentrated hydrochloric acid, stir at room temperature for 1h, filter with suction, cool the filtrate to 0-5°C, add acetone dropwise until a large amount of turbidity appears, keep stirring for 1h, continue to add 600mL of acetone dropwise, keep warm Stir for 1 h, filter and dry with suction to obtain 48 g of white cefotiam hydrochloride crystalline powder, with a molar yield of 85%, an HPLC content of 98.12%, and a maximum simple compound of 0.46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com