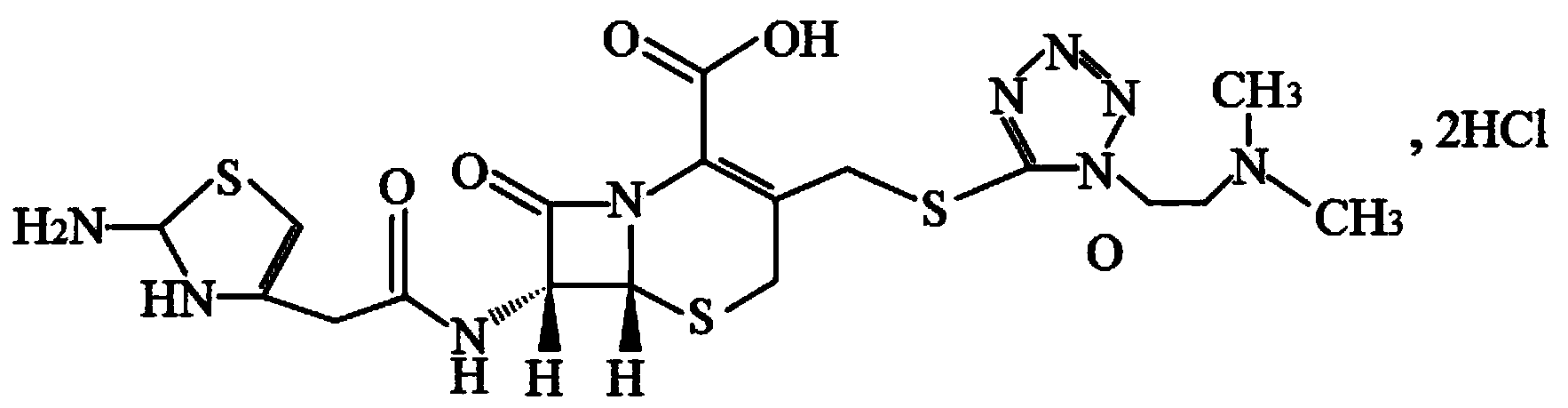
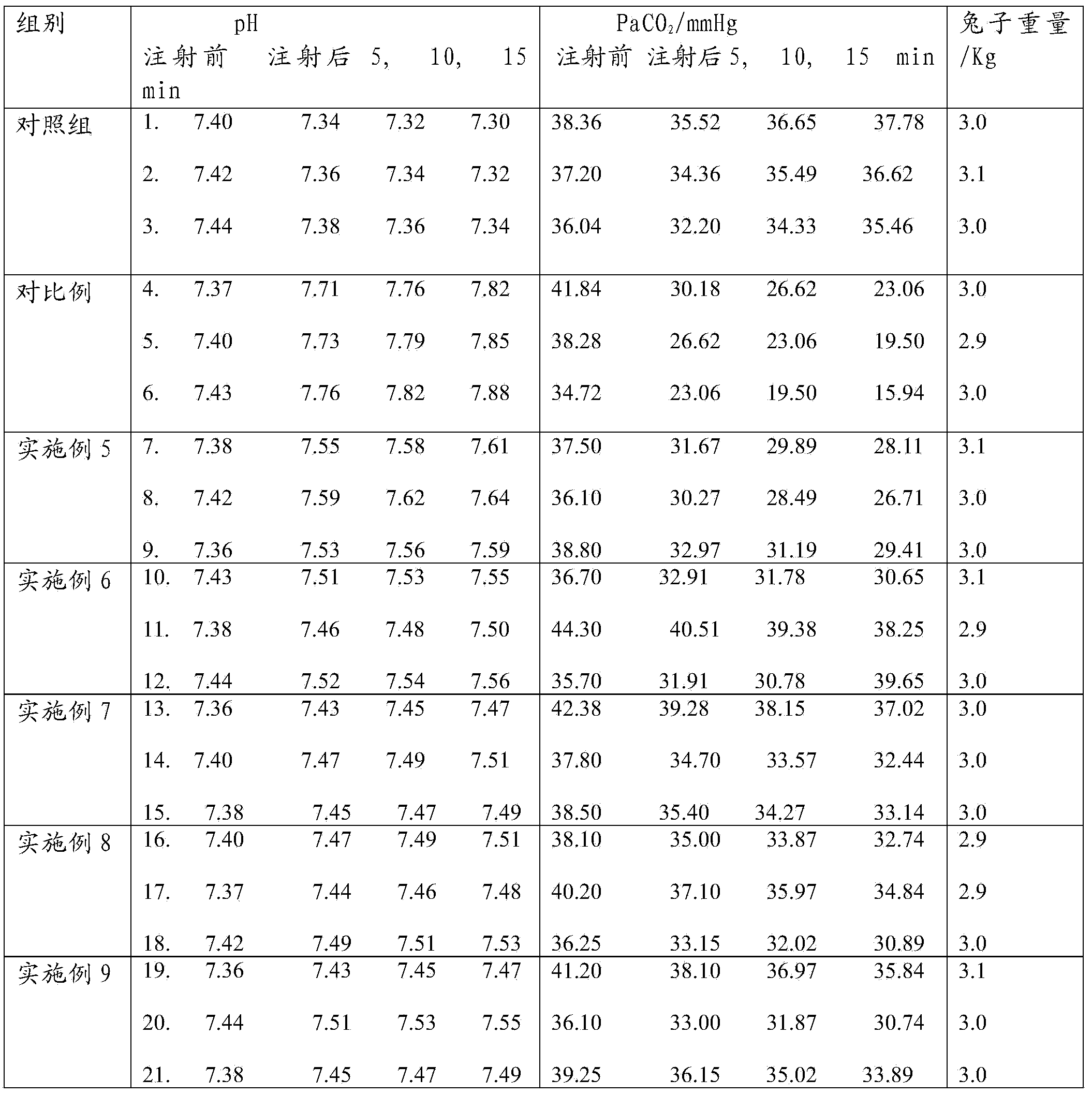
Cefotiam hydrochloride pharmaceutical composition for injection and preparation method thereof
A technology for cefotiam hydrochloride and a composition, which is applied in the field of drug synthesis and preparation, can solve problems such as poisoning, poor pH stability, and excessively low partial pressure, and achieves the effects of good stability, enhanced pH stability, and small variation range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The pharmaceutical composition of cefotiam hydrochloride for injection described in the present embodiment, its formula is as follows:
[0077] Cefotiam hydrochloride 500g, sodium carbonate 120g, sodium bicarbonate 2g;
[0078] The pharmaceutical composition is prepared by the following steps:
[0079] (1) Filling. After mixing cefotiam hydrochloride with sodium carbonate / sodium bicarbonate, the filling is filled into sterilized vials using an automatic filling machine, and the vials are sterilized using a tunnel sterilizer. The sterilization temperature is 320 degrees;
[0080] (2) Capping and adding a sterilized rubber stopper, the rubber stopper is autoclaved at 121 degrees for 20 minutes, and then dried at 50 degrees for 2 hours;
[0081] (3) Sealing, cover the aluminum cover on the rubber plug;
[0082] (4) Visual inspection.
Embodiment 2
[0084] The pharmaceutical composition of cefotiam hydrochloride for injection described in the present embodiment, its formula is as follows:
[0085] Cefotiam hydrochloride 500g, sodium carbonate 118g, sodium bicarbonate 6g;
[0086] The pharmaceutical composition is prepared by the following steps:
[0087] (1) Filling. After mixing cefotiam hydrochloride with sodium carbonate / sodium bicarbonate, the filling is filled into sterilized vials using an automatic filling machine, and the vials are sterilized using a tunnel sterilizer. The sterilization temperature is 320 degrees;
[0088] (2) Capping and adding a sterilized rubber stopper, the rubber stopper is autoclaved at 121 degrees for 20 minutes, and then dried at 50 degrees for 2 hours;
[0089] (3) Sealing, cover the aluminum cover on the rubber plug;
[0090] (4) Visual inspection.
Embodiment 3
[0092] The pharmaceutical composition of cefotiam hydrochloride for injection described in the present embodiment, its formula is as follows:
[0093] Cefotiam hydrochloride 500g, sodium carbonate 116g, sodium bicarbonate 10g;
[0094] The pharmaceutical composition is prepared by the following steps:
[0095] (1) Filling. After mixing cefotiam hydrochloride with sodium carbonate / sodium bicarbonate, the filling is filled into sterilized vials using an automatic filling machine, and the vials are sterilized using a tunnel sterilizer. The sterilization temperature is 320 degrees;
[0096] (2) Capping and adding a sterilized rubber stopper, the rubber stopper is autoclaved at 121 degrees for 20 minutes, and then dried at 50 degrees for 2 hours;
[0097] (3) Sealing, cover the aluminum cover on the rubber plug;
[0098] (4) Visual inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com