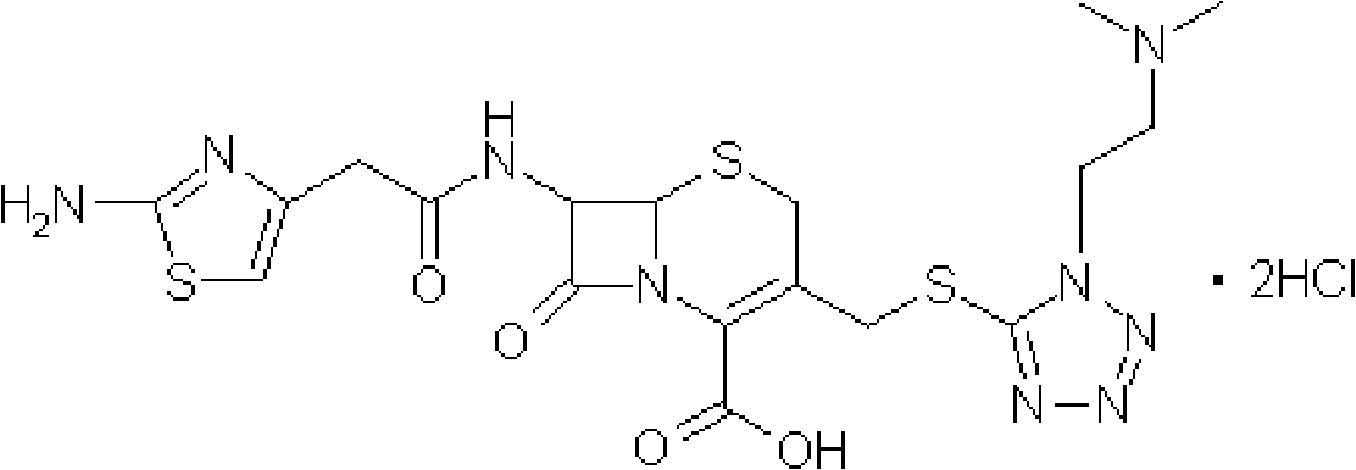
Hydrochloric acid cefotiam crystalline compound, preparation method thereof and medicine combination containing compound
A technology of cefotiam hydrochloride and crystal compounds, applied in antibacterial drugs, organic chemistry, pharmaceutical formulations, etc., can solve the problems of increased risk of allergic reactions, increased polymer content, etc., to improve drug safety and Effectiveness, incidence reduction, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
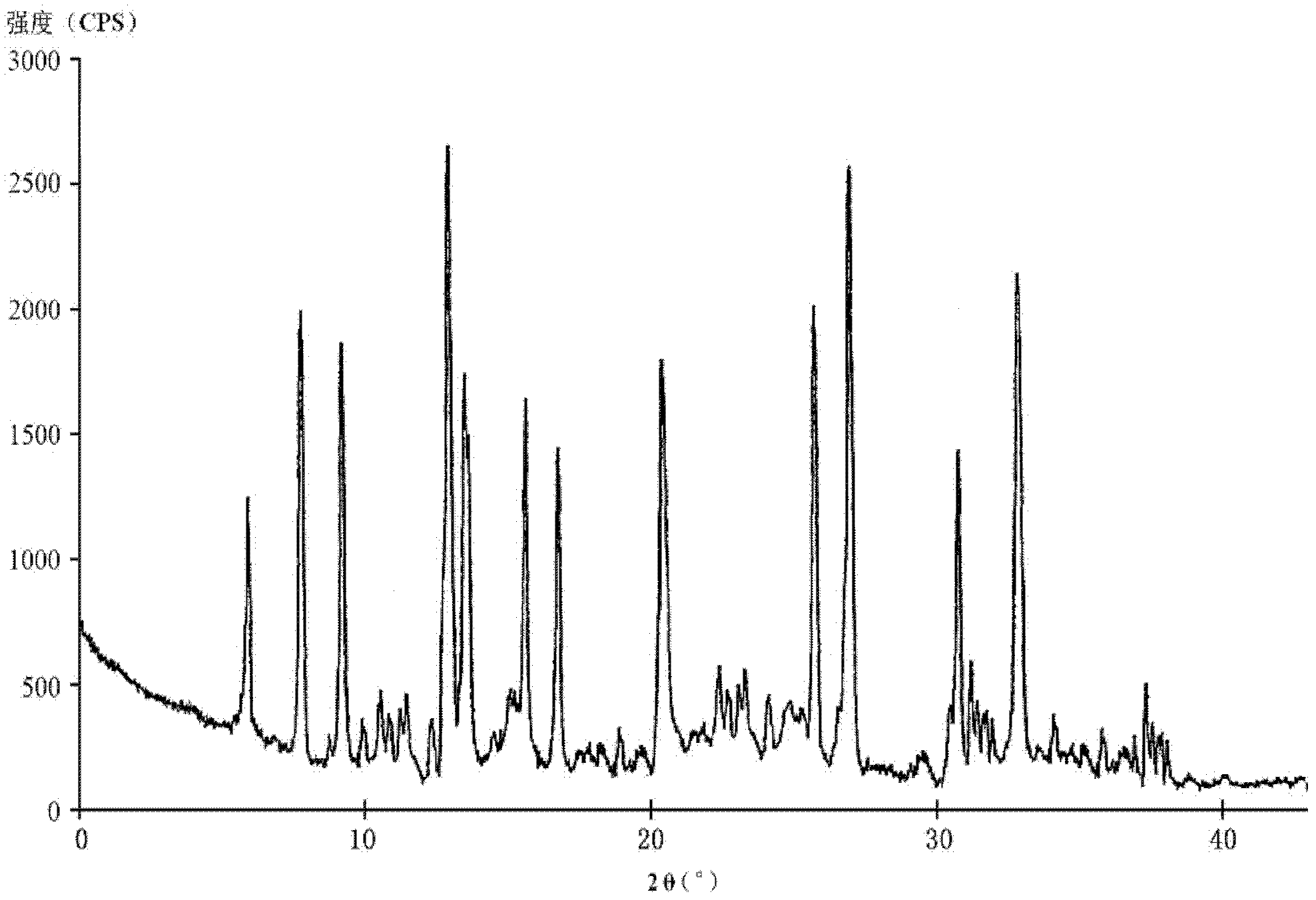
[0042] [embodiment 1] the preparation of cefotiam hydrochloride crystal
[0043] 1) Prepare the crude product solution: add 200 g of the crude product of cefotiam hydrochloride into 1500 ml of a mixed solvent prepared by methanol and tetrahydrofuran in a volume ratio of 5:1, stir to dissolve, and add 0.1% of the crude product of cefotiam hydrochloride by weight Activated charcoal decolorizes, filters, and obtains the crude product solution, for subsequent use;
[0044] 2) Preparation of crystallization solvent: prepare a crystallization solvent with acetone and ethyl acetate in a volume ratio of 2:10, and the volume of the crystallization solvent is 12 times the weight of the crude product of cefotiam hydrochloride;
[0045]3) Crystallization: under stirring, add the crystallization solvent obtained in step 2) to the crude product solution obtained in step 1), and solids are precipitated; after the addition is completed, continue to add chloroform dropwise under stirring until...
Embodiment 2-9
[0049]
[0050] The crystalline compound of cefotiam hydrochloride obtained in Examples 2-9 was measured by powder X-ray diffraction method, and the X-ray powder diffraction pattern represented by 2θ±0.2° diffraction angle was the same as in Example 1.
preparation Embodiment 1
[0051] [Preparation Example 1] Cefotiam Hydrochloride Sterile Powder for Injection
[0052] Specification:
[0053] Preparation:
[0054] 1. Inner packaging material treatment
[0055] Antibiotic glass bottles, rubber stoppers, and aluminum caps are cleaned, dried, and sterilized according to the conventional process, and set aside;
[0056] 2. Specific steps
[0057] (1) take by weighing the anhydrous sodium carbonate of recipe quantity and the prepared cefotiam hydrochloride crystal of embodiment 1, mix homogeneously in aseptic container;
[0058] (2) Inspection of intermediate products;
[0059] (3) Carry out aseptic subpackaging according to specifications;
[0060] (4) vacuuming, plugging and capping;
[0061] (5) Packaging, full inspection, and storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com