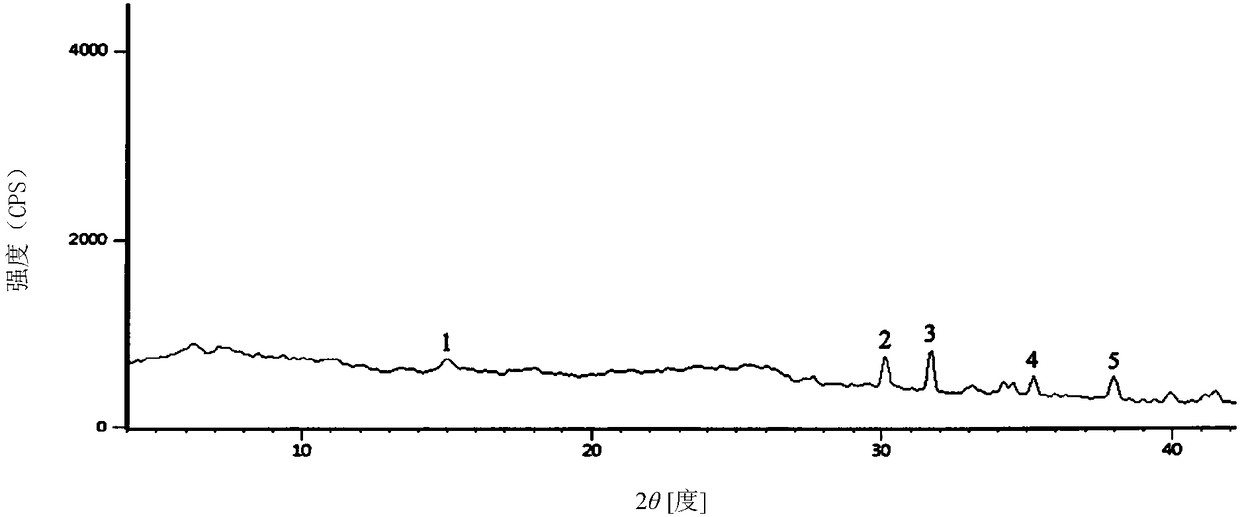
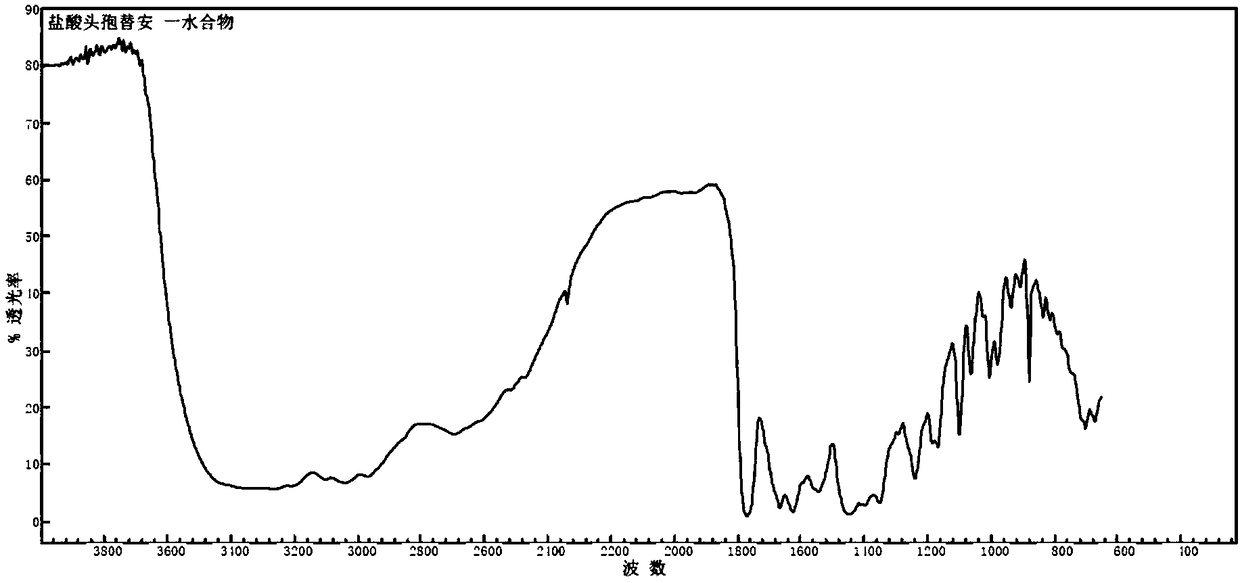
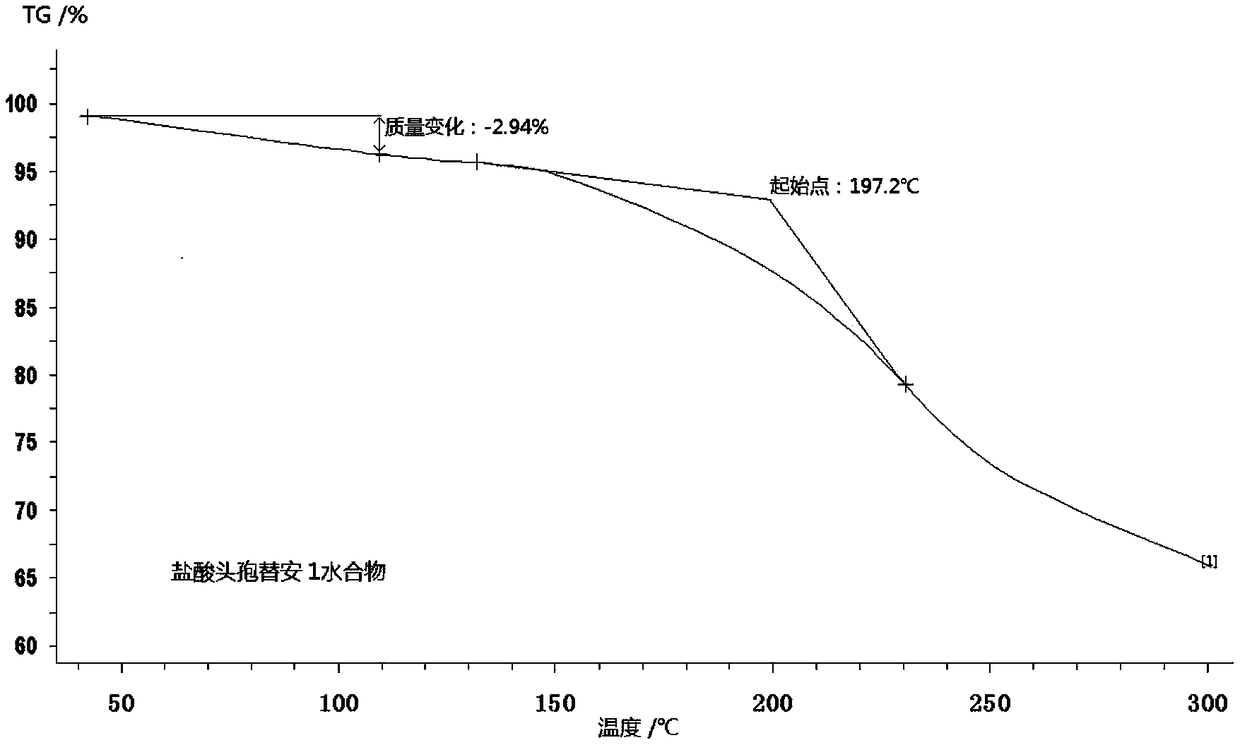
Monohydrater cefotiam hydrochloride compound and pharmaceutical composition thereof
A technology of cefotiam hydrochloride and cephalosporin hydrochloride, which is applied in the field of medicine and chemical industry, and can solve the problems of difficult control of reaction, difficult operation and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 monohydrate cefotiam hydrochloride compound
[0040] making process
[0041] (1) Add 200ml of dichloromethane to the three-necked flask of the reactor, after cooling down to -10°C, add aminothiazole acetate hydrochloride, then add 38g of phosphorus trichloride to react for 2h, filter, and wash with dichloromethane to obtain white wet spare parts;
[0042] (2) Add 250ml of dimethyl carbonate into another three-necked flask of the reactor, cool to 10°C, and slowly introduce BF under stirring 3 Gas 110g (about 1.0h), make the boron trifluoride dimethyl ester solution for subsequent use;
[0043] (3) Add 80g of 7-ACA, 40g of 1-(2-dimethylaminoethyl)-5-mercaptotetrazole, and 250ml of dimethyl carbonate to another dry three-necked flask, stir and react for 10min, and cool down to 10 ℃, add the above-prepared boron trifluoride dimethyl carbonate solution, raise the temperature to 50 ℃ and react for 1.0h, after the reaction is complete, cool t...
Embodiment 2
[0048] The preparation of embodiment 2 monohydrate cefotiam hydrochloride compound
[0049] making process
[0050] (1) Add 400ml of dichloromethane to the three-neck flask of the reactor, after cooling down to -5°C, add aminothiazole acetate hydrochloride, then add 75g of phosphorus trichloride to react for 3.0h, filter, wash with dichloromethane to get white Wet product standby;
[0051] (2) Add 500ml of dimethyl carbonate into another three-necked flask of the reactor, cool to 15°C, and slowly introduce BF under stirring 3 Gas 220g (about 1.5h), the prepared boron trifluoride dimethyl ester solution is standby;
[0052] (3) Add 160g of 7-ACA, 80g of 1-(2-dimethylaminoethyl)-5-mercaptotetrazole, and 500ml of dimethyl carbonate to another dry three-necked flask, stir for 20min, and cool down to 20 ℃, add the above-prepared boron trifluoride dimethyl carbonate solution, raise the temperature to 45 ℃ and react for 1.5h, after the reaction is complete, cool the reaction solut...
Embodiment 3
[0057] The preparation of embodiment 3 monohydrate cefotiam hydrochloride compound
[0058] making process
[0059] (1) Add 300ml of dichloromethane to the three-necked flask of the reactor, after cooling down to 0°C, add aminothiazole acetate hydrochloride, then add 58g of phosphorus trichloride to react for 2.5h, filter, wash with dichloromethane to obtain a white wet spare parts;
[0060] (2) Add 520ml of dimethyl carbonate into another three-necked flask of the reactor, cool to 20°C, and slowly introduce BF under stirring 3 Gas 170g (about 1.0h), the prepared boron trifluoride dimethyl ester solution is standby;
[0061] (3) Take another dry three-necked flask and add 7-ACA 120g, 1-(2-dimethylaminoethyl)-5-mercaptotetrazole 60g, dimethyl carbonate 375ml into it, stir for 15min, cool down to 15 ℃, add the boron trifluoride dimethyl carbonate solution prepared above, raise the temperature to 40 ℃ and react for 1.5h, after the reaction is completed, the temperature of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com