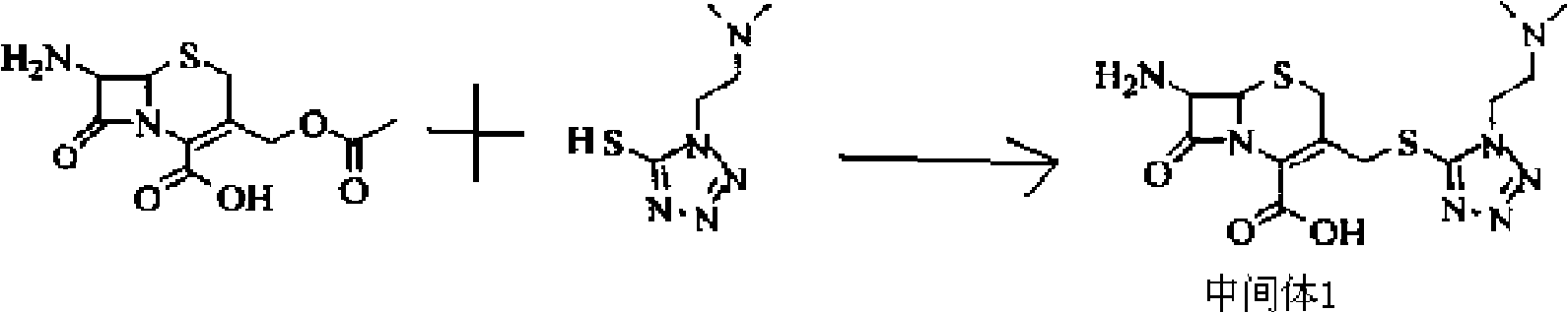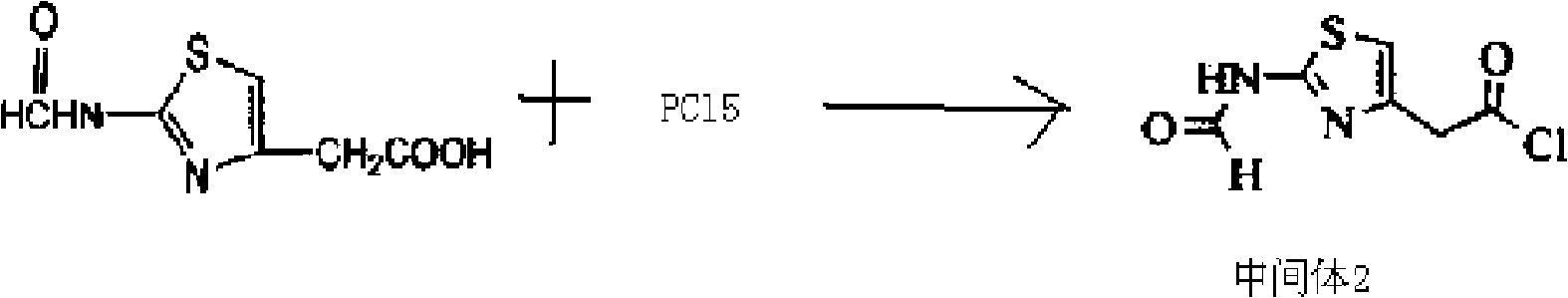Cefotiam hydrochloride medicament composition sterile powder injection and preparation method thereof
A technology of cefotiam hydrochloride and sterile powder injection, which is applied in the field of drug synthesis and preparation, can solve the problems of low yield, low product yield and high solvent residue, and achieves low content of related substances, high purity and stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
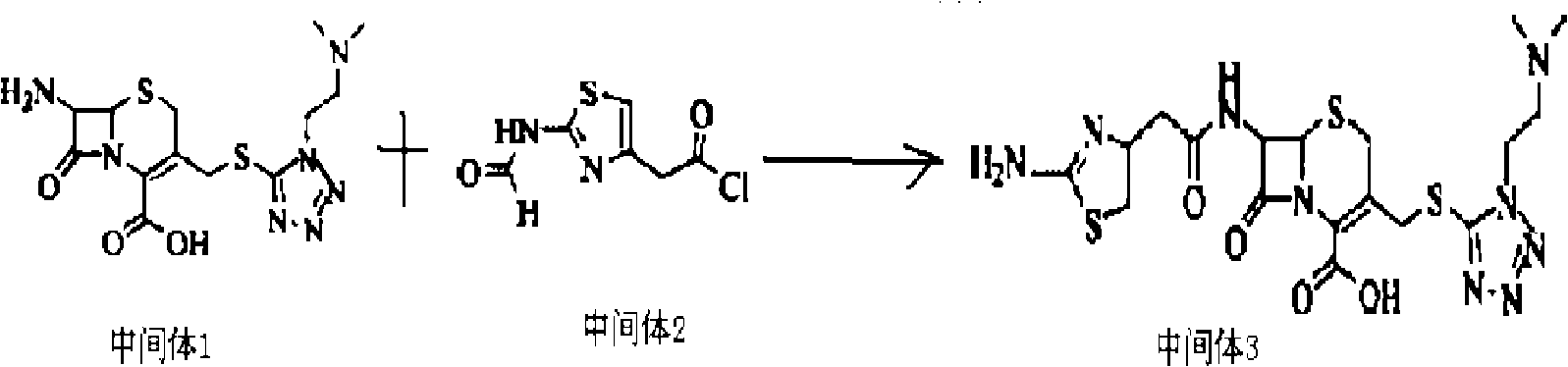
[0056] (1) Weigh 27.2 grams of 7-ACA and add it to 140 mL of anhydrous acetonitrile to form a suspension; add 17.3 grams of 5-mercapto-1-dimethylamino-ethyl-1H to the formed suspension -tetrazolium to form a mixed solution, and when the temperature of the mixed solution was kept at -5 to 5°C, 160 mL of boron trifluoride-diethyl ether complex (containing 20 grams of boron trifluoride, 0.3 mol, mixed at 30 React at ℃ for 2.5 hours, then cool to 5℃, add 30mL of 37% hydrochloric acid dropwise, adjust the pH value to 3.0 with 28% ammonia water in an ice bath, grow crystals for 1 hour, filter, wash the filter cake with 50ml of acetone, and set at 45℃ After drying, 33.1 g of intermediate 1 were obtained.
[0057] (2) Dissolve 18.6 grams of 2-formylamino-thiazolyl-4-acetic acid in 250 mL of dichloromethane, cool to -20 ° C, add PCl in batches, stir while adding, the stirring speed is 130 rpm Minutes, after the addition was complete, continue to stir for 2 hours at a stirring speed of...
Embodiment 2
[0062] (1) Weigh 27.2 grams of 7-ACA and add it to 140 mL of anhydrous acetonitrile to form a suspension; add 25.95 grams of 5-mercapto-1-dimethylamino-ethyl-1H to the formed suspension -tetrazolium to form a mixed solution. When the temperature of the mixed solution was kept at 0-5°C, 170 mL of boron trifluoride-diethyl ether complex (containing 23 grams of boron trifluoride, 0.35 mol) was added, and the mixture was mixed uniformly at 38 React at ℃ for 2 hours, then cool to 5℃, add 28mL of 37% hydrochloric acid dropwise, adjust the pH value to 3.0 with 25% ammonia water in an ice bath, grow crystals for 1.5 hours, filter, wash the filter cake with 50ml of acetone, and store at 40℃ After drying, 32.8 g of intermediate 1 were obtained.
[0063] (2) Dissolve 18.6 grams of 2-formylamino-thiazolyl-4-acetic acid in 250 mL of dichloromethane, cool to -20°C, add PCl in batches, stir while adding, the stirring speed is 120 rpm minutes, after the addition was complete, the stirring wa...
Embodiment 3
[0068] (1) Weigh 27.2 grams of 7-ACA and add it to 140 mL of anhydrous acetonitrile to form a suspension; add 13.84 grams of 5-mercapto-1-dimethylamino-ethyl-1H to the formed suspension -tetrazolium to form a mixed solution. When the temperature of the mixed solution was kept at -2 to 2°C, 160 mL of boron trifluoride-diethyl ether complex (containing 20 grams of boron trifluoride, 0.3 mol, mixed at 30 React at -38°C for 2-2.5 hours, then cool to 2°C, add 37% hydrochloric acid dropwise, adjust the pH value to 3.0 with 27% ammonia water in an ice bath, grow crystals for 1 hour, filter, and wash the filter cake with 50ml acetone , 33.1 g of Intermediate 1 were obtained after drying at 45 °C.
[0069] (2) Dissolve 18.6 grams of 2-formylamino-thiazolyl-4-acetic acid in 250 mL of dichloromethane, cool to -20 ° C, add PCl in batches, stir while adding, the stirring speed is 110 rpm Minutes, after the addition was complete, continue to stir for 3 hours at a stirring speed of 60 rpm, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com