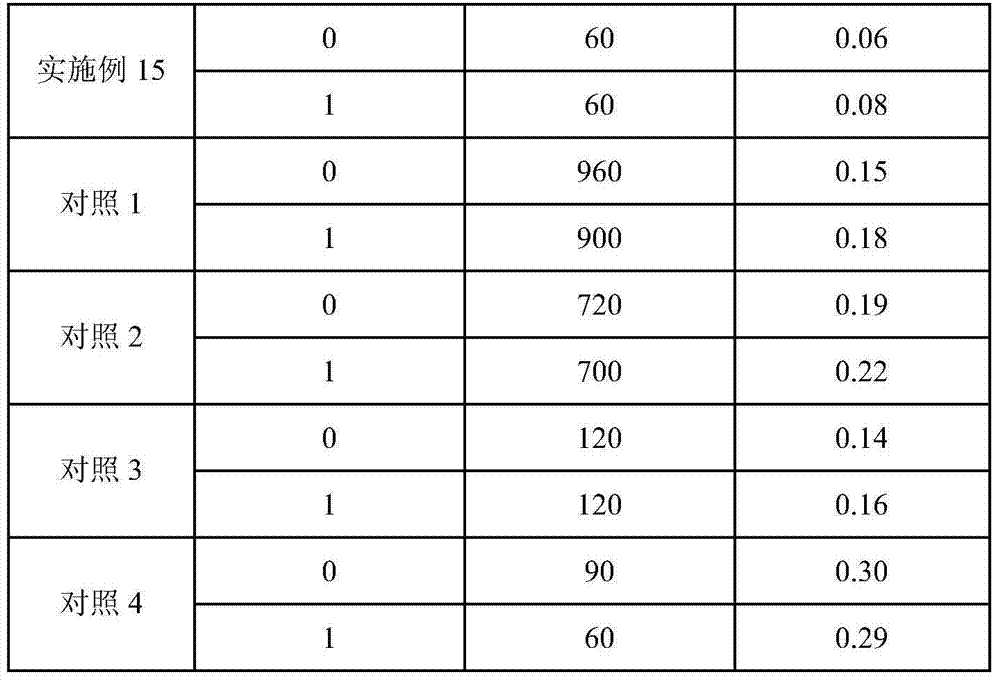
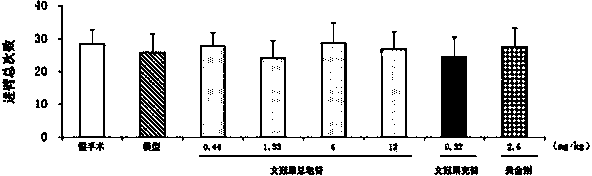
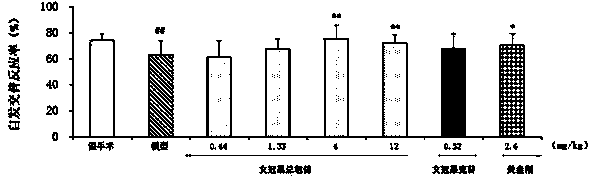
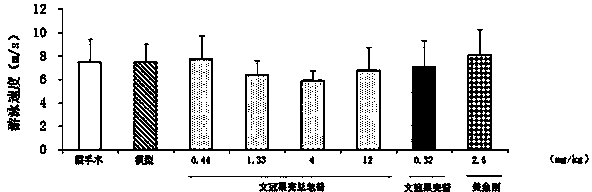
Patents
Literature
42results about How to "Low organic residue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
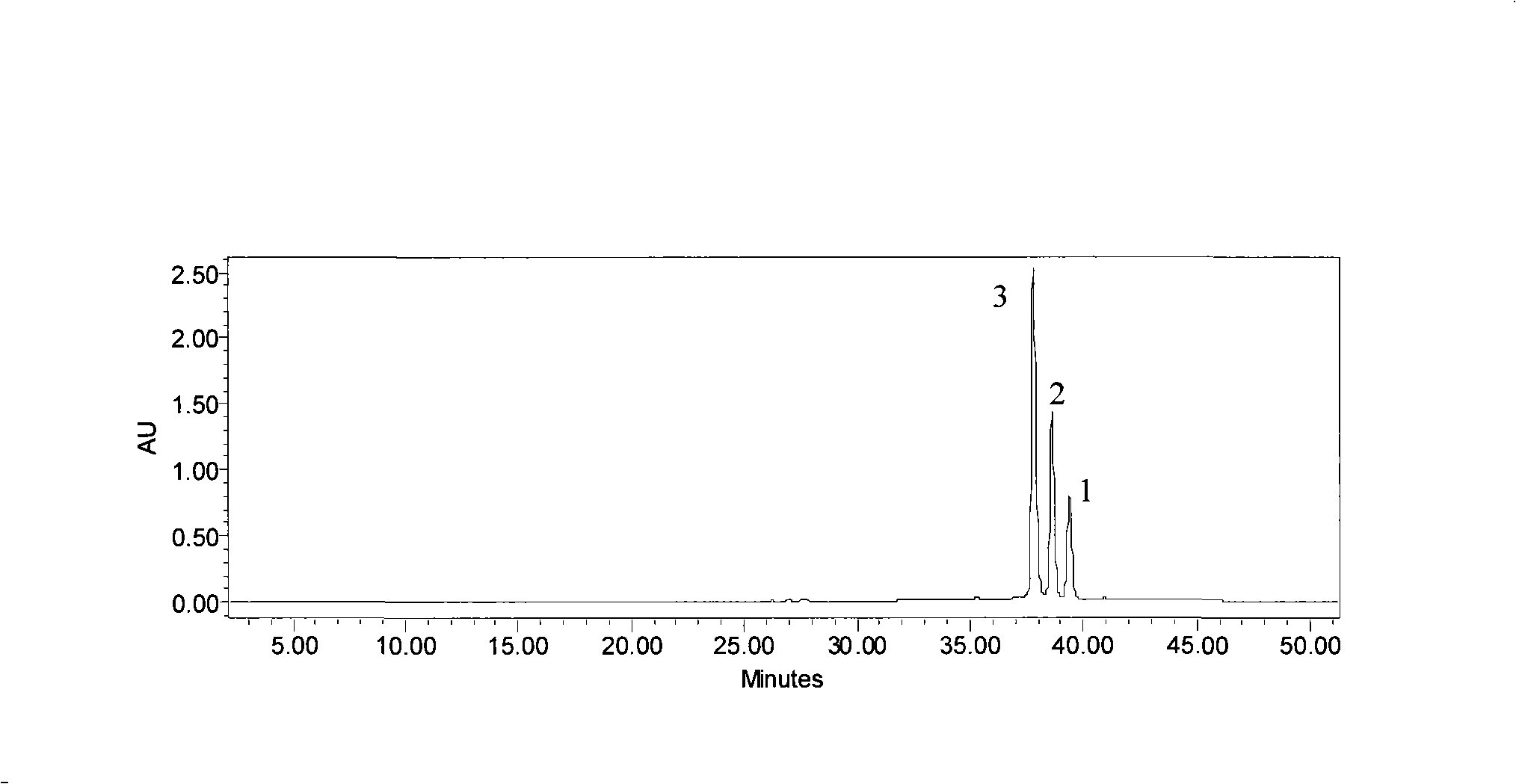
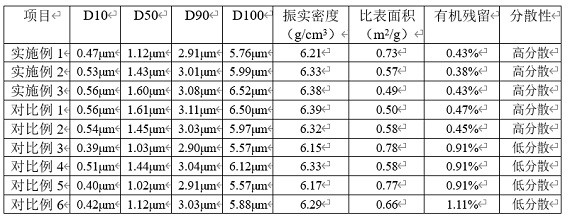
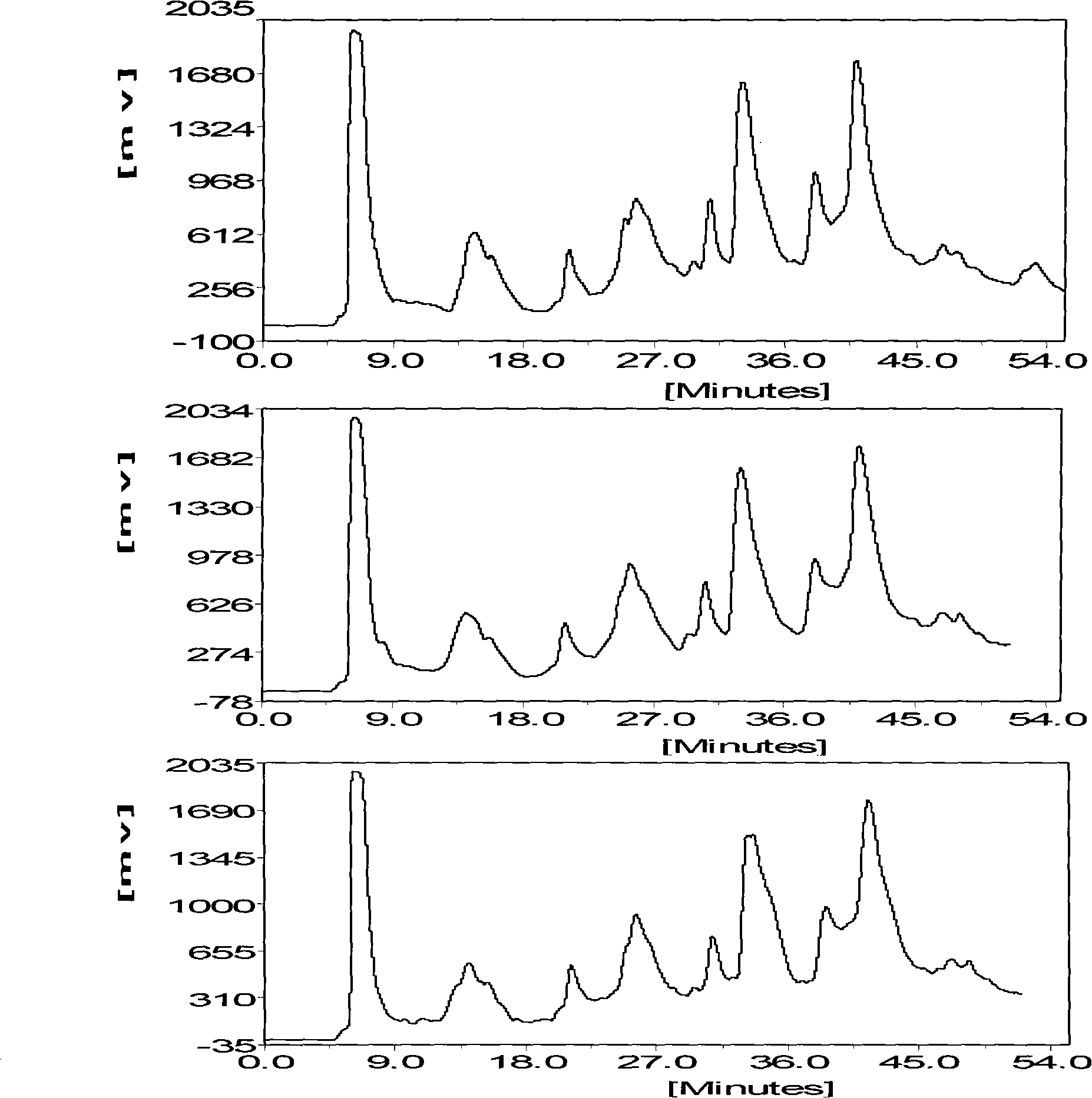
Extraction technique of high-purity phlorizin
InactiveCN101392008ARaw materials are easy to getSimple extraction processSugar derivativesSugar derivatives preparationCentrifugationGradient elution
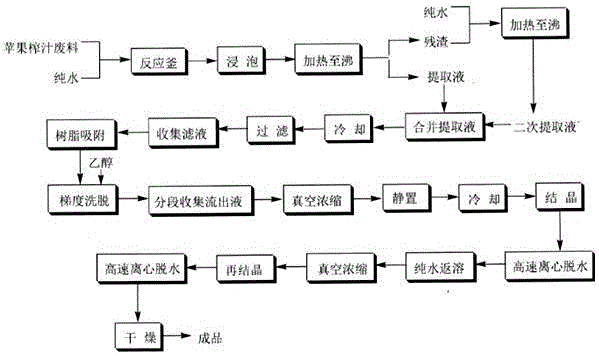
The invention discloses an extraction process of a high purity phlorizin, comprising the following steps: (1) the raw materials are added with 4-8 times pure water, marinated for 1-3 hours, heated to boiling and micro boiling is kept for 2-3 hours so as to obtain an extracting solution; residue is heated to boiling by using 2-6 times pure water and micro boiling is kept for 2-3 hours so as to obtain a secondary extracting solution; the extracting solutions are merged, cooled and filtered and then the filtered liquid is collected; (2) the filtered liquid is adsorbed by macroporous resin and subjected to gradient elution by using 20 DEG and 50 DEG ethanol; the effluent liquid is collected in subsection, concentrated in vacuum, placed in a quiescence way and cooled, dissolves out crystal at 0-4 DEG C, and dehydrates by high-speed centrifugation; and (3) re-dissolving of pure water, vacuum concentration, re-crystallization, high-speed centrifugation dehydration and drying are carried out so as to obtain the finished product. By adopting pure water for extraction, the organic-residue is decreased and simultaneously the product content is more than 99 percent.
Owner:XINGHUA GL STEVIA CO LTD
Method for preparing amorphous atorvastatin calcium
The invention discloses a method for preparing amorphous Atorvastatin calcium. The current method uses more solvent, so the cost is over high; and the residual quantity of the solvent in a product is large, thereby causing big influence on the quality of the product and causing serious environmental pollution. In the method, alcohol solvent and water are combined into mixed alcohol-water solvent which dissolves Atorvastatin calcium containing one or more crystal forms completely; proper temperature is kept for ensuring that the Atorvastatin calcium is not precipitated; and the amorphous Atorvastatin calcium is precipitated by a spray drying method. The method uses the mixed alcohol-water solvent which is removed from the product easily, has little organic residue and causes less influence on drug quality; the usage amount of the solvent is reduced greatly; the concentration of the Atorvastatin calcium in the solution is high; and the purity of the prepared product of the amorphous Atorvastatin calcium is high.
Owner:ZHEJIANG JINGXIN PHARMA
Hedyotis diffusa Willd. extract and method for separating and preparing the same
InactiveCN101496845AIncrease contentGuaranteed purityDigestive systemAntiviralsChromatographic separationMacroporous resin
The invention relates to a spreading hedyotis herb extract and a separation and preparation method thereof. The main components of the extract are paederoside methyl ester, deacetylasperulosidic acid methyl ester, asperulosidic acid and asperuloside, and the total content of the four main components is 60 to 75 percent. The extract is obtained from the spreading hedyotis herb through steps of extracting, depositing in alcohol, membrane separation, non-polar macroporous resin separation, industrial chromatographic resolution, and the like. The preparation method can effectively remove the impurities such as glycoprotein, polysaccharide, amino acid, and the like, and improve the content of the main active components. The preparation process has high repeatability and good operability, is easy to realize standardization and industrialization, and simultaneously has certain directive significance for the quality control of the spreading hedyotis herb.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for purifying chenodeoxycholic acid
The invention discloses a method for purifying chenodeoxycholic acid. The method comprises the following steps: pretreatments such as degreasing, decoloration and impurity removal are carried out on commercially available crude bile paste or crude bile powder, so as to obtain a component to undergo chromatographic separation; the component to undergo chromatographic separation undergoes purification and separation by the use of a chromatographic column with a hydrophilic resin filler as a stationary phase; and separation products undergo concentration, acidification, washing and drying, so as to obtain chenodeoxycholic acid. In comparison with the prior art, the invention has the following beneficial effects: 1, content of the main component is high; 2, reappearance is high and operability is good; 3, short cycle: it only takes two days to prepare the high-purity chenodeoxycholic acid product from processing of the bile paste raw material; and 4, low content of organic residues: as processes such as solvent extraction, column chromatography on silica gel and the like in the traditional techniques are abandoned, an industrial chromatographic process with low dosage of an organic solvent is adopted and the final product undergoes drying process, the content of the residual organic solvent in the final product is especially low.
Owner:SHANGHAI FENPU NEW MATERIAL TECH CO LTD
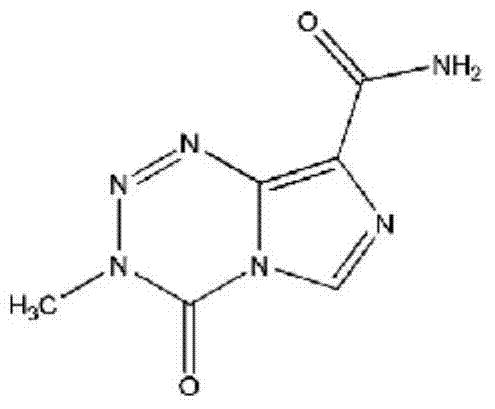
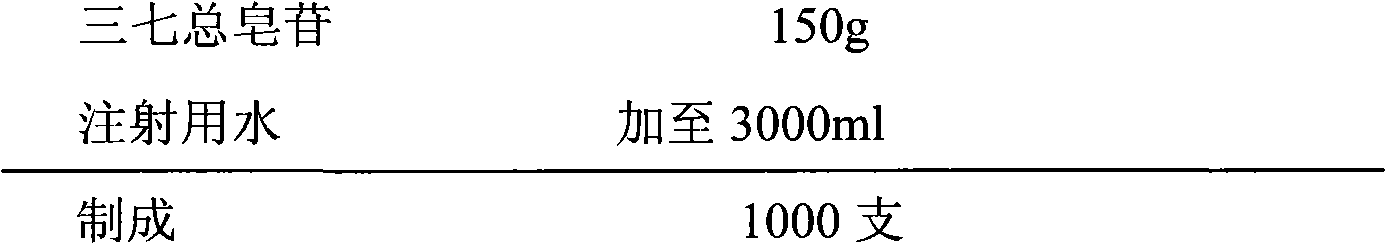
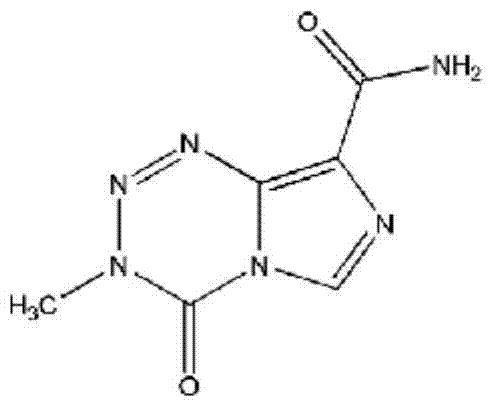
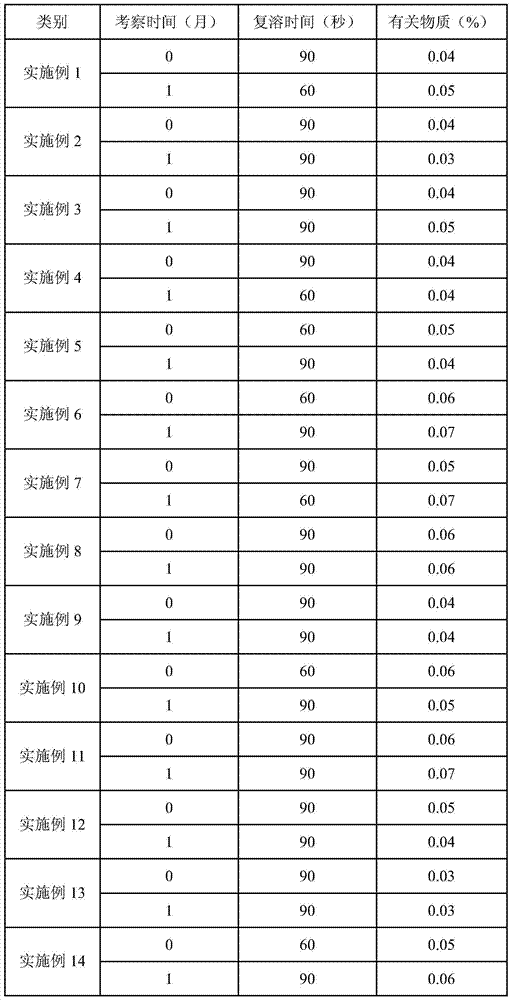
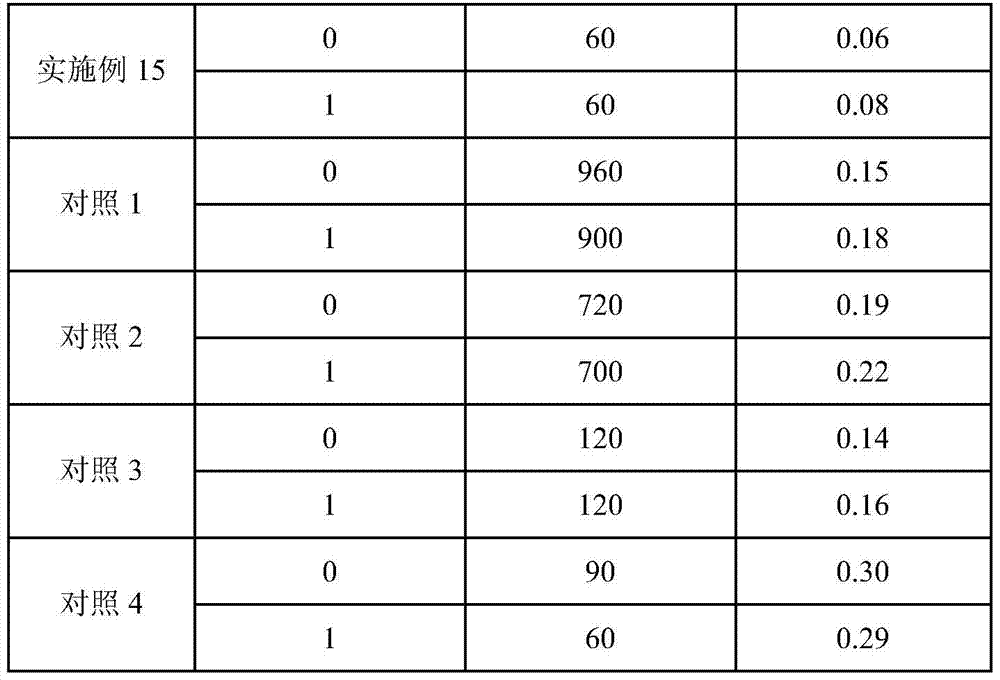
Temozolomide lyophilized powder preparation and preparation method thereof
ActiveCN104721155AReduce adverse reactionsLow toxicityOrganic active ingredientsPowder deliveryDissolutionBULK ACTIVE INGREDIENT
The invention belongs to the technical field of pharmaceutical preparations and specifically relates to a temozolomide lyophilized powder preparation. The temozolomide lyophilized powder preparation comprises an active ingredient of temozolomide or a pharmaceutically acceptable salt thereof, and a solution before lyophilization further contains an excipient, a wetting agent, a buffer agent, an osmotic pressure regulating agent, a pH regulating agent, water for injection and an organic solvent, wherein the organic solvent is selected from one or any combination of ethanol, acetone, isopropanol, n-propanol, butanone, sec-butyl alcohol and methanol, and is preferably ethanol. The temozolomide lyophilized powder preparation provided by the invention has the advantages of stable quality, high re-dissolution speed and a small residual amount of the organic solvent. The invention further provides a method for preparing the preparation. The process provided by the invention is simple and convenient in preparation process and easy to control production links, the organic solvent accounts for a relatively small part of total volume of material liquid, the pollution to production equipment and environment caused by the organic solvent is reduced, and thus the method is suitable for large-scale production.
Owner:QILU PHARMA HAINAN
Purification method of cefotiam hydrochloride and aseptic powder injection of cefotiam hydrochloride
ActiveCN102746324AHigh yieldLow organic residueAntibacterial agentsOrganic active ingredientsPurification methodsNitrogen gas
The invention relates to the technical field of medicaments, and particularly relates to a purification method of cefotiam hydrochloride and an aseptic powder injection of cefotiam hydrochloride. The method comprises the steps of: dissolving cefotiam sodium hydrochloride crude product with water, adding activated carbon for injection to remove pyrogen, then adding acetone, recrystallizing, washing obtained crystal with ethyl ether or methyl tert-butyl ether, filtering, carrying out suction filtering water-saturated nitrogen gas, drying under reduced-pressure to obtain cefotiam aseptic powder which is low in organic residue, pyrogen-free and high in purity.
Owner:HAINAN JINXING PHARMA
Method for preparing shiny-leaved yellowhorn total saponins and application of shiny-leaved yellowhorn total saponins
ActiveCN103830412ALow costReduce lossesNervous disorderFood preparationCerebral injuryCerebral arterial thrombosis
The invention belongs to the technical field of medicines, and relates to a novel method for preparing shiny-leaved yellowhorn total saponins from shiny-leaved yellowhorn and an application of the shiny-leaved yellowhorn total saponins. According to the method, a coarse extract of the shiny-leaved yellowhorn is directly separated and purified by a macroporous resin method to obtain shiny-leaved yellowhorn total saponins; the yield of the total saponins is over 5 percent, and the content of the total saponins in a product is over 60 percent and far higher than that in products prepared by other processes. The prepared shiny-leaved yellowhorn total saponins can be applied to preparation of medicines or health-care products for preventing and treating memory deterioration, hypophrenia and cranial nerve injury caused by neurodegenerative diseases and acute and chronic cerebral injury diseases, and can be used for treating cerebral diseases caused by alzheimer disease, acute disease and subacute stage of cerebral arterial thrombosis, vascular dementia and other ischemia and hypoxia of neurodegenerative diseases and acute and chronic cerebral injury diseases.
Owner:SHENYANG PHARMA UNIVERSITY
High efficiency extraction method of T-acid mother liquor
ActiveCN105129894ASave energy costsSave production costNature of treatment waterWater/sewage treatment by extractionInorganic saltsKerosene
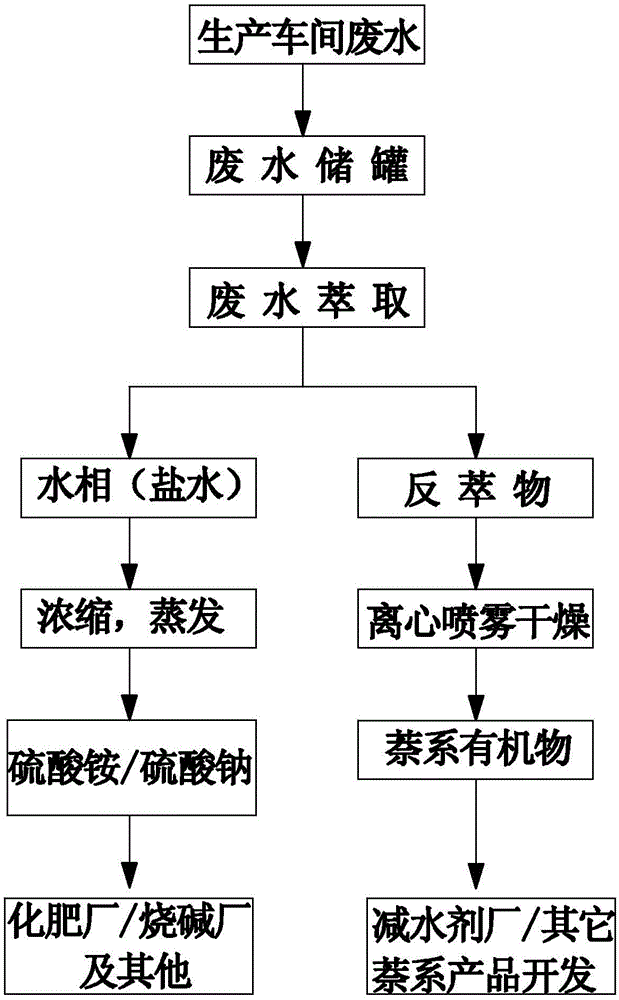
The invention relates to a high efficiency extraction method of T-acid mother liquor, and belongs to the field of an H-acid production waste water treatment. Tri(octyl-decyl)amine, sulfonated kerosene and n-octyl alcohol are respectively measured and uniformly mixed for obtaining an extractant, the extractant is thrown into the T-acid mother liquor, a heating stirring is carried out, simultaneously sulfuric acid is added, after stirring, standing and layering, the upper layer is an extract, and lower layer is an inorganic salt water phase; after the lower layer inorganic salt water phase is separated, the upper layer extract is heated, sodium hydroxide is added, after stirring and standing, the oil and water separation is carried out. The invention is applied into the H-acid production, thereby avoiding generation of solid waste with simple operation and effectively reducing the production cost.
Owner:JIANGXI MADE FINE CHEM IND
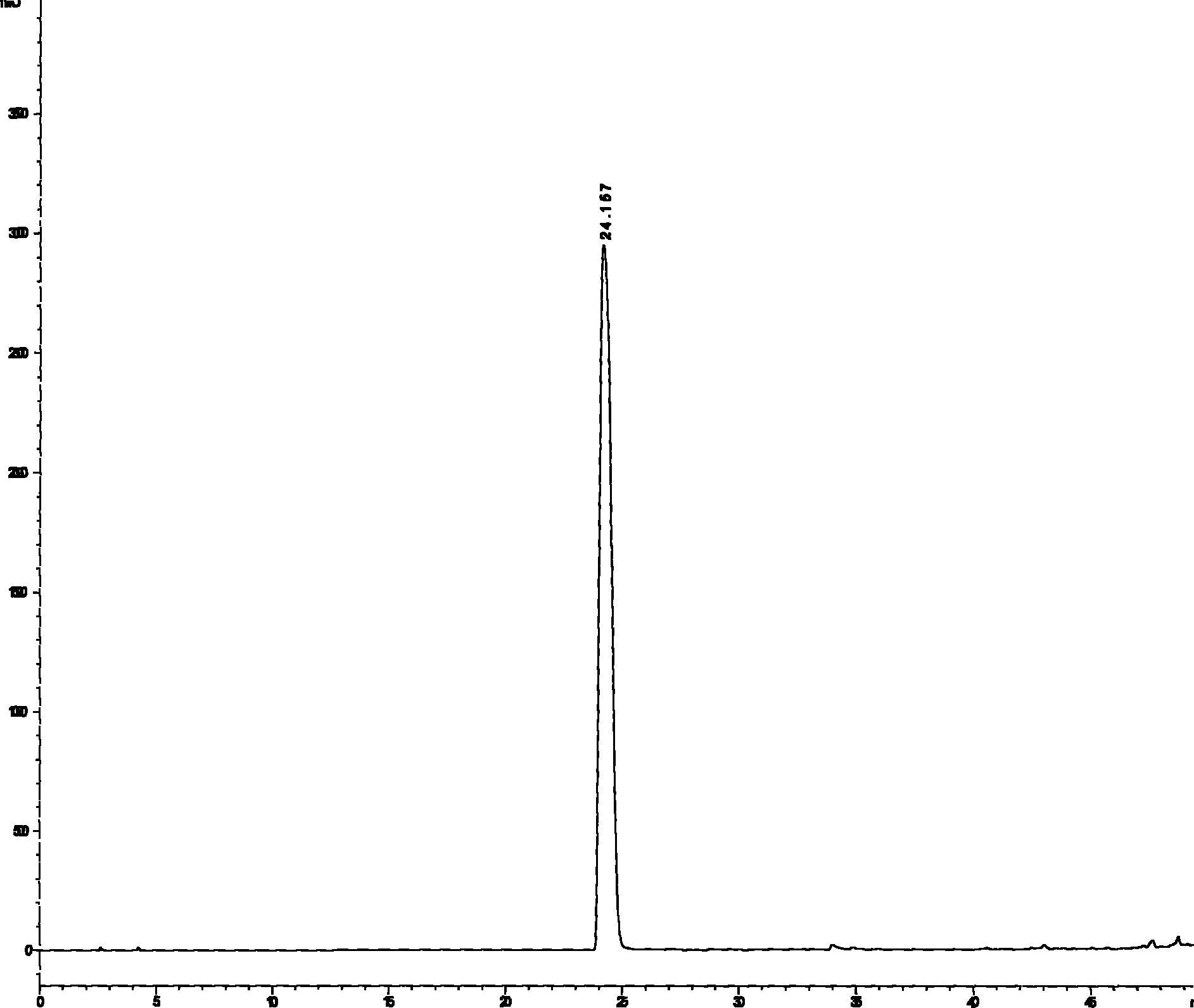
Extraction process of high-purity phloridzin
InactiveCN106336441ASolve the real problemNo pollution in the processSugar derivativesSugar derivatives preparationGradient elutionMacroporous resin
The invention discloses an extraction process of high-purity phloridzin. The process includes the steps of: (1) stock solution extraction: adding water that is 8 times the amount of a phloridzin-containing mashed raw material into the same, conducting soaking for 3h, performing heating to boiling, maintaining slight boiling for 2h to obtain an extracted solution; adding purified water that is 6 times the amount of residue into the same and performing heating to boiling, maintaining slight boiling for 2h to obtain a secondary extracted solution; merging the extracted solutions, carrying out cooling and filtering, and collecting the filtrate; (2) preliminary extraction of a finished product: subjecting the filtrate to adsorption by macroporous resin D101, controlling the column pass flow at 80L / H, using 20-50degree ethanol to conduct gradient elution, performing fractional collection on the outflow solution, carrying out vacuum concentration, standing and cooling, conducting crystal precipitation at 0-4DEG C, and carrying out high-speed centrifugal dehydration; and (3) finished product extraction: conducting pure water redissolution, vacuum concentration, recrystallization, high-speed centrifugal dehydration and drying, thus obtaining a finished product. The extraction process of high-purity phloridzin provided by the invention has easily available raw materials, not only solves the environmental problem, but also improves the product yield, and greatly reduces the cost, and has the advantages of simple reaction process, convenient operation and no pollution.
Owner:TIANJIN UNIQUE FRUITS & VEGETABLES FOOD CO LTD
Treatment process combining H acid mother solution and T acid mother solution
PendingCN105130079AReduce solid wasteEnsure healthy and sustainable developmentAmmonium sulfatesMultistage water/sewage treatmentInorganic saltsPre treatment
The invention relates to a treatment process combining an H acid mother solution and a T acid mother solution and belongs to the technical field of treatment of H acid production wastewater. The treatment process comprises the following steps: adding an acidic complexing agent into collected wastewater and pre-treating; extracting an obtained water phase; directly adding the water phase into MVR equipment, and concentrating and crystallizing; centrifuging and separating to obtain inorganic salt; adding sodium hydroxide into an extracting solution except the water phase and adjusting temperature and pH to carry out reverse extraction; separating a reverse extraction product obtained by the reverse extraction from an extraction agent, and drying the reverse extraction product to obtain an organic byproduct. The treatment process is applied to the production of H acid, so that the production of solid wastes is avoided and the operation is simple and convenient; recycled products also can be put into secondary utilization so that the production cost is effectively reduced.
Owner:JIANGXI MADE FINE CHEM IND
Preparation for effective component of turmeric
InactiveCN101317997AGuaranteed purityIncrease contentAntipyreticMetabolism disorderChromatographic separationMethanol water
The present invention provides a method for producing turmeric effective component. Turmeric material is ground and added with 70 to 95 percent of ethanol for extraction; the turmeric material is loaded on nonpolar macroporous adsorptive resin and ethanol / water is used for eluting; the eluent is collected, concentrated and dried to obtain turmeric macroporous resin component; then turmeric effective component is obtained through industrial production of chromatographic separation and mobile phase of methanol water. The main active ingredients are curcumin, demethoxycurcumin and bisdemethoxycurcumin and the total content is up to 97.3 percent. The turmeric effective component produced by the present invention gets rid of impurities and increases the content of the main active ingredients. In production, the present invention has high repeatability, good maneuverability, easy standardization and industrialization. At the same time, the present invention has certain directive significance for quality control of turmeric herb.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for separating dichloropropanol from dichloropropanol and hydrochloric acid azeotrope
ActiveCN109232183ADifficult to separate directlyBig difference in boiling pointOrganic compound preparationHydroxy compound preparationDistillationEther
The invention discloses a method for separating dichloropropanol from dichloropropanol and hydrochloric acid azeotrope. The method specifically comprises the following steps: adding arenes, esters andethers which are azeotropic with water as an organic water-carrying agent to the dichloropropanol and hydrochloric acid azeotrope to form a new azeotropic system, heating and enabling the system to be azeotropic, through rectifying, cooling and distributing water, realizing separation of hydrochloric acid and the organic water-carrying agent; performing atmospheric distillation on an unrectifiedcomponent containing dichloropropanol to obtain the high-purity dichloropropanol, and recovering and recycling the water-carrying agent of the system. The method is simple in operation, high in separating efficiency of dichloropropanol and hydrochloric acid solution, high in recovery rate, and high in purity of the obtained dichloropropanol and hydrochloric acid. The organic water-carrying agent of arenes, esters and ethers is high in water-carrying efficiency, recycled, and low in cost, and capable of reducing environment pollution.
Owner:SHANDONG TAIHE WATER TREATMENT TECH CO LTD
A kind of purification method of Rhodorin
InactiveCN102286043AHigh purityEasy to separateSugar derivativesSugar derivatives preparationChromatographic separationCountercurrent chromatography
The invention discloses a method for purifying Rhododendron, which is characterized in that: the round leaves of Rhizoma chinensis are used as raw materials, crushed, and 55-70% ethanol is added for ultrasonic extraction 1-3 times, the extract is concentrated under reduced pressure, and the concentrated solution is adjusted to pH 3- 4. Filtrate, add polyamide resin to the filtrate for adsorption, elute with ethanol water, concentrate the collected solution to recover ethanol, filter the concentrated solution through a high-speed centrifuge, and separate the filtrate through a membrane separator. The obtained components are separated by high-speed countercurrent chromatography and detected by an ultraviolet detector , collect the target components, and dry under reduced pressure to obtain Rhodorin. The method of the invention has the advantages of simple operation, short production period, large preparation amount and high efficiency, and is suitable for the preparation of high-purity rhodium.
Owner:NANJING ZELANG MEDICAL TECH
Method for extracting total saponins from fructus gleditsiae
The invention relates to a method for extracting total saponins from fructus gleditsiae, which comprises the following steps of: firstly, crushing fructus gleditsiae dried medicinal material, adding 5-8 volumes by weight of lower alcohol water solution, carrying out ultrasound extraction for 2-3 times and filtering to obtain an extraction solution; secondly, carrying out condensation under reduced pressure on the extraction solution until no alcohol flavor exists, adding the extraction solution in a macroporous resin column, washing with water or 10-20 percent ethanol solution to remove impurities, eluting with 55-70 percent of ethanol solution and collecting an eluent; and thirdly, reducing the pressure and recovering the ethanol from the eluent, filtering through an ultrafiltration membrane, condensing filtrate through a nanofiltration membrane and drying to obtain the total saponins from the fructus gleditsiae. The method has the advantages of less pollution, low cost and high yield; and the obtained product has no solvent residue and can be directly used for producing foods and medicines.
Owner:NANJING ZELANG AGRI DEV
Xanthoceraside preparation method
The invention belongs to the field of medical technology and relates to a new method for preparing xanthoceraside in shinyleaf yellowhorns. The highly purified xanthoceraside can be prepared efficiently with the preparation method. The preparation method is easy and convenient to use and reliable and reduces redundant intermediate operation steps, thus, product loss is reduced, and the yield of the preparation method is obviously increased compared with that of a former method. Crude extract is directly processed with a silica gel column, eluted with the chloroform / dichloromethane-methyl alcohol eluent with the volume ratio of 10:1-7:1, the ethyl acetate-methyl alcohol eluent with the volume ratio of 10:1-5:1 or the acetone-methyl alcohol eluent with the volume ratio of 10:1-6:1, and then eluted with the chloroform / dichloromethane-methyl alcohol eluent with the volume ratio of 6:1-2:1, the ethyl acetate-methyl alcohol eluent with the volume ratio of 4:1-1:1 or the acetone-methyl alcohol eluent with the volume ratio of 5:1-3:1, solvent recovery, purification and recrystallization are performed, and finally the xanthoceraside is obtained.
Owner:SHENYANG PHARMA UNIVERSITY
A treatment process for resource utilization of medical waste salt by medium-temperature pyrolysis
ActiveCN111468516BFully oxidative decompositionLow organic residueSolid waste disposalTransportation and packagingInorganic saltsAnticaking agent
The present invention relates to the field of environmental protection, and specifically relates to a treatment process for resource utilization of medical waste salt at medium temperature pyrolysis; the present invention provides a process for resource utilization of waste medical salt at medium temperature pyrolysis. The principle is to use lower temperature to fully oxidize and decompose organic pollutants from the waste salt that has removed volatile substances, so as to achieve the purpose of harmless treatment of waste salt and then resource utilization; the invention adds an inorganic salt anti-caking agent, It can effectively prevent the waste salt from agglomerating in the fluidized bed reactor and ensure the sufficient oxidation and decomposition of organic pollutants, which is the key to the process; the waste salt obtained by the treatment method of medical waste salt of the present invention has extremely low organic residues and no agglomeration phenomenon. The organic matter is fully oxidized, and the energy saving is significantly reduced. The effective treatment of medical waste salt can not only protect the environment, but also reuse the main component sodium chloride salt to make up for the market gap, which has high economic value.
Owner:浙江红狮环保股份有限公司
Trollius chinensis extract, preparation method thereof and preparation containing same
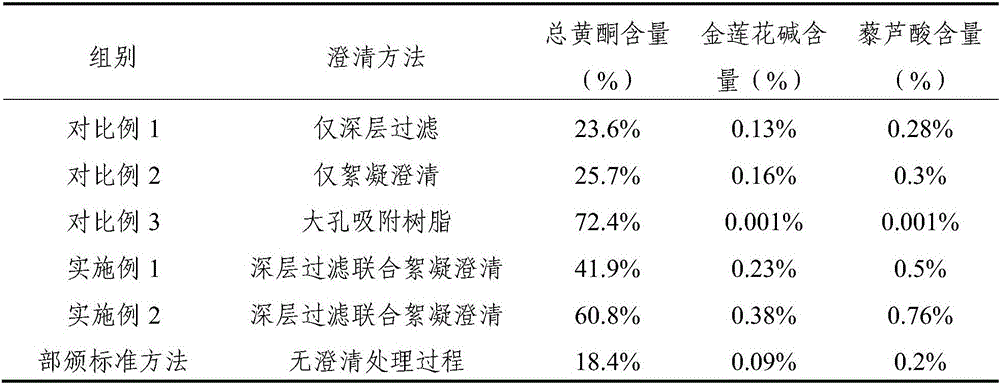
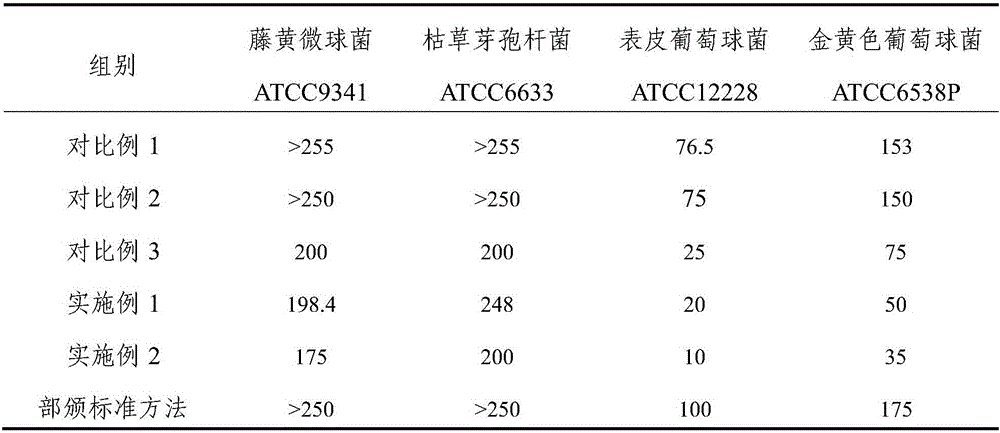
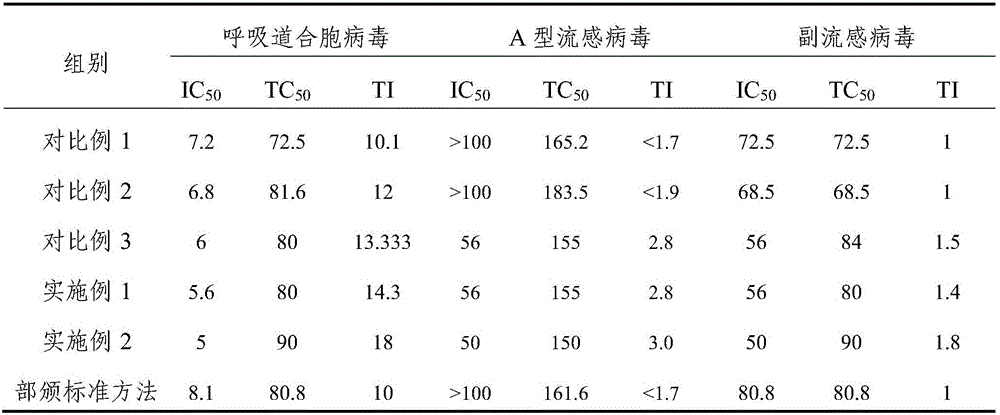
ActiveCN105998215AEfficient removalHigh content of active ingredientsAntibacterial agentsSenses disorderFiltrationAntibacterial activity
The invention relates to a trollius chinensis extract, a preparation method thereof and a preparation containing the same. The trollius chinensis extract contains total flavone, cytisine and veratric acid, and the preparation method of the trollius chinensis extract includes the steps that trollius chinensis liquid obtained through water decoction or alcohol water is subjected to deep filtration, and a flocculating and clarifying agent is adopted for clarification; the flocculating and clarifying agent is a type-II ZTCl + natural clarifying agent or is prepared from a component B in the type-II ZTCl + natural clarifying agent and chitosan. A deep filtration combined flocculation precipitation technology is adopted to remove impurities, macromolecular substances such as tannin are effectively removed, the flavone, the cytosine, the veratric acid and other active ingredients are also effectively retained, the active ingredient content of the extract is improved, and the antibacterial activity and in-vitro antiviral activity of the extract are strong. The preparation method is simple, capable of saving energy, environmentally friendly and low in organic residue content.
Owner:李宏 +1
Preparation method and application of indocyanine green polymer nano particles
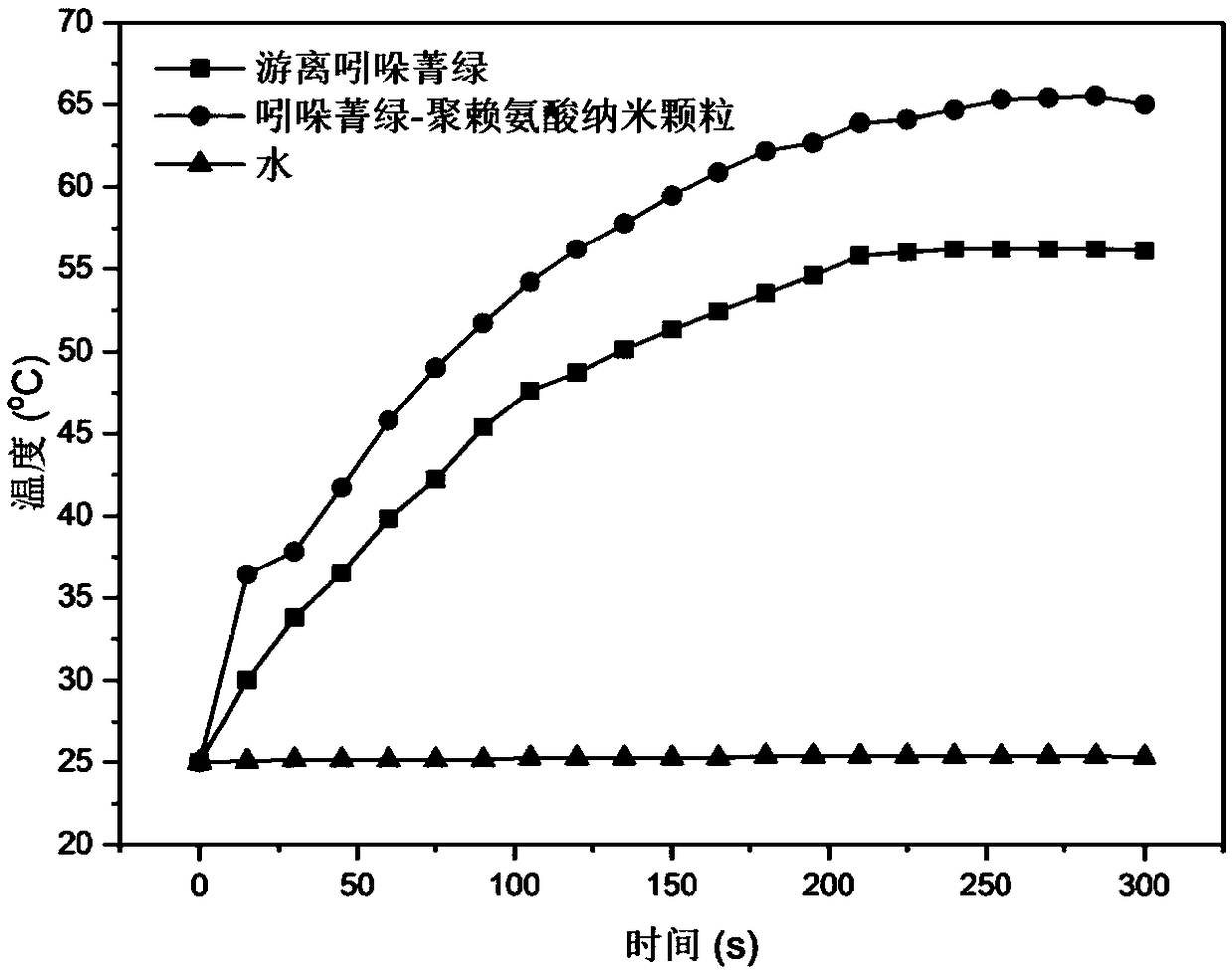
ActiveCN108853498ASimple processShort preparation cyclePowder deliveryEnergy modified materialsOrganic solventFreeze-drying
The invention discloses a preparation method and application of indocyanine green polymer nano particles. The preparation method comprises the following steps: (1) dissolving indocyanine green in a first organic solvent, dropwise adding a second organic solvent, and obtaining a mixture; (2) pumping the mixture into a supercritical granulation autoclave, and after the mixture is pumped, continuously flushing by using supercritical carbon dioxide, thus obtaining the indocyanine green nano particles; and (3) ultrasonically dispersing the indocyanine green nano particles in a sodium chloride solution comprising polycations, stirring, then centrifuging, washing obtained precipitates by using ultrapure water, and then freeze drying, thus obtaining the indocyanine green polymer nano particles. The indocyanine green polymer nano particles prepared in the invention are good in stability, an optical absorption wavelength range is at a near-infrared waveband, the high penetration performance of the near-infrared rays for biological tissues can be well utilized, and the indocyanine green polymer nano particles can be better used for treating tumors.
Owner:HUAQIAO UNIVERSITY
Preparation method of silver powder
ActiveCN114367674ADispersedUniform particle sizeTransportation and packagingMetal-working apparatusFluid phaseHydroxyl ion
According to the scheme, a liquid phase reduction method is firstly adopted, silver nitrate and a reducing agent are adopted according to the main principle, and a certain amount of water-based dispersing agent or oil-based dispersing agent is added, so that the silver powder with certain dispersing performance is obtained, and in order to guarantee that the silver powder with high dispersity and uniform particle size can be obtained, the preparation method is simple, and the cost is low. The water-based dispersing agent is selected, meanwhile, the reducing agent is any one or more of ascorbic acid, formaldehyde, hydrazine hydrate and hydrogen peroxide, after the reduction reaction is finished, alkali liquor continues to be added, the pH of the suspension C is adjusted to be 8-14, hydroxyl ions are contained in the system in the process, the structure of the dispersing agent can be destroyed through strong alkalinity, and therefore the dispersing effect is improved. Therefore, the dissociation effect is achieved; the method can be applied to any preparation method of the silver powder using the water-based dispersing agent, complete sedimentation of the silver powder can be achieved, organic residues on the surface of the obtained silver powder are lower than those of the silver powder not using the method by 0.3% or above, and high practicability is achieved.
Owner:南通领跑者新材料科技有限公司 +1
Environment-friendly cooling liquid for cutting hard brittle material, as well as preparation method and using method thereof
ActiveCN105969480AImprove wettabilityHigh specific heat capacity characteristicsWorking accessoriesFine working devicesCutting forceSolvent
The invention discloses environment-friendly cooling liquid for cutting a hard brittle material, as well as a preparation method and a using method thereof. Based on the total amount of 100 weight percent, the environment-friendly cooling liquid contains the following components in percentage by weight: 30 to 50 percent of a cleaning agent, 10 to 30 percent of a nonionic surfactant, 2 to 10 percent of a wetting agent, 15 to 30 percent of an emulsifier and 0 to 10 percent of a solvent. According to the environment-friendly cooling liquid, as well as the preparation method and the using method thereof, lubrication effects in a cutting process are enhanced, and a cutting tool is favorably protected, so that cutting force is increased; in addition, low-foam effects are maintained in the whole cutting process, so that cutting is facilitated, the undesirable phenomena of edge breakage and the like of a cut product are avoided, and the cutting yield is greatly improved.
Owner:辽宁晟新科技股份有限公司
Method for separating and preparing salidroside
Owner:浙江华谱新创科技有限公司
Functional silver flake and preparation method thereof
The invention discloses a preparation method of functional silver flakes, which includes the following steps of (A), preparing microcrystalline micro-silver powder containing silver oxide by means of primary reduction; (B), grinding silver flakes with a ball mill and then filtering the same to obtain silver slurry containing ball-milled dispersant; (C), treating the silver powder by etching and secondary reduction to obtain stereoscopic cross-linked structural silver flakes with 3-12% micro-silver powder, wherein a reducing agent A is an alcohol weak reductant while a reducing agent B is a strong reductant. The preparation method is simple in process, easy to realize mass production and applicable to large-scale production in the industry. The functional silver flakes prepared by the method have stereoscopic cross-linked reticular space structures, small in grain size and low in apparent density. Besides, since the functional silver flakes contain 3-10% of monocrystal or flocculent silver powder, point-surface contact among the silver powder and filling capability of the silver powder are improved, and conductivity is high.
Owner:广东羚光新材料股份有限公司
A kind of preparation method and application of total saponins of Xingguan fruit
ActiveCN103830412BLow costReduce lossesNervous disorderCardiovascular disorderCerebral arterial thrombosisCerebral injury
The invention belongs to the technical field of medicines, and relates to a novel method for preparing shiny-leaved yellowhorn total saponins from shiny-leaved yellowhorn and an application of the shiny-leaved yellowhorn total saponins. According to the method, a coarse extract of the shiny-leaved yellowhorn is directly separated and purified by a macroporous resin method to obtain shiny-leaved yellowhorn total saponins; the yield of the total saponins is over 5 percent, and the content of the total saponins in a product is over 60 percent and far higher than that in products prepared by other processes. The prepared shiny-leaved yellowhorn total saponins can be applied to preparation of medicines or health-care products for preventing and treating memory deterioration, hypophrenia and cranial nerve injury caused by neurodegenerative diseases and acute and chronic cerebral injury diseases, and can be used for treating cerebral diseases caused by alzheimer disease, acute disease and subacute stage of cerebral arterial thrombosis, vascular dementia and other ischemia and hypoxia of neurodegenerative diseases and acute and chronic cerebral injury diseases.
Owner:SHENYANG PHARMA UNIVERSITY
A kind of method separating dichloropropanol from dichloropropanol hydrochloric acid azeotropic liquid
ActiveCN109232183BLow organic residueEasy to operateOrganic compound preparationHydroxy compound preparationPropanolDistillation
Owner:SHANDONG TAIHE WATER TREATMENT TECH CO LTD
Method for preparing hydrophilic drug microsphere through spray drying process
ActiveCN102228440BRound shapeFlat surfaceOrganic active ingredientsPharmaceutical non-active ingredientsMicrosphereOil phase
The invention relates to the technical field of preparation of hydrophilic drug microspheres, in particular discloses a method for preparing a hydrophilic drug microsphere through a spray drying process. The method comprises preparation steps of: uniformly dispersing hydrophilic drug powder into an isolation oil phase to form an S / O1 type suspension, and then slowly adding into a spray solution (O2) which contains a macromolecular carrier, an anti-sticking agent and a plasticizing agent, homogeneously emulsifying to form an S / O1 / O2 type spray emulsion, decompressing and drying at 60 DEG C or less through a spray drying technology after collecting the microspheres in a cyclone separator, and screening with a sieve of 80 meshes so as to prepare the hydrophilic drug twice-embedded sustained-release microsphere. The microsphere prepared by spray drying have the advantages of round shape, smooth surface, good mobility, uniform grain size distribution, high encapsulating rate, capability ofeffectively controlling burst release, and accordance with the characteristics of a long-acting preparation; and the method has the advantages of reducing consumption of a great quantity of oil substances and volatile organic solvents , greatly shortening drying time and being remarkable in effect, and is applicable to industrial production.
Owner:武汉回盛生物科技股份有限公司
Amorphous cefodizime sodium and preparation method thereof and pharmaceutical composition comprising same
InactiveCN101747345AEasy to operateImprove liquidityAntibacterial agentsOrganic active ingredientsCefodizime SodiumX-ray
The invention provides an amorphous cefodizime sodium, which is characterized in that an amorphous X-ray diffraction pattern is free from having an X-ray diffraction peak; and in an amorphous differential thermogram, the endothermic peak stays at 57 to 68 DEG C, and the exothermic peak stays at 250 to 258 DEG C. The organic residual value in the cefodizime sodium is low, so the cefodizime sodium is free from entering into a hard lamp during the drying and is easy to operate; the obtained product presents in an apparent granular shape, and the fluidity is good; and when the cefodizime sodium is used for the preparation, the cefodizime sodium is free from being crushed and can be directly homogenized to be prepared, thereby saving device, power and labor cost for the industrialized production, and shortening the production period.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Method for purifying panax notoginseng total saponins and preparation of panax notoginseng total saponins
ActiveCN102526147BHigh purityGood decolorizationMetabolism disorderImmunological disordersPANAX NOTOGINSENG ROOTActivated carbon
The invention relates to a method for purifying panax notoginseng total saponins and a preparation of the panax notoginseng total saponins, and aims to provide a purifying method capable of preparing panax notoginseng total saponins with higher efficiency and higher purity and overcoming the defects of the prior art. The purifying method breaks through the conventional method for purifying the panax notoginseng total saponins; impurity removal, decolorization and enrichment of the total saponins can be effectively implemented by fully combining different purifying effects of macroporous resin, polyamide and active carbon; and the polyamide is filtered by one step of washing operation, so the process is simple, an organic solvent is saved and industrialized production is easily realized. The invention also comprises the medicinal preparation of the panax notoginseng total saponins, and the preparation can be prepared into power injection, injection, tablets or capsules.
Owner:北京中海康医药科技发展有限公司
A kind of temozolomide freeze-dried powder preparation and preparation method thereof
ActiveCN104721155BReduce adverse reactionsLow toxicityPowder deliveryOrganic active ingredientsDissolutionBULK ACTIVE INGREDIENT
The invention belongs to the technical field of pharmaceutical preparations and specifically relates to a temozolomide lyophilized powder preparation. The temozolomide lyophilized powder preparation comprises an active ingredient of temozolomide or a pharmaceutically acceptable salt thereof, and a solution before lyophilization further contains an excipient, a wetting agent, a buffer agent, an osmotic pressure regulating agent, a pH regulating agent, water for injection and an organic solvent, wherein the organic solvent is selected from one or any combination of ethanol, acetone, isopropanol, n-propanol, butanone, sec-butyl alcohol and methanol, and is preferably ethanol. The temozolomide lyophilized powder preparation provided by the invention has the advantages of stable quality, high re-dissolution speed and a small residual amount of the organic solvent. The invention further provides a method for preparing the preparation. The process provided by the invention is simple and convenient in preparation process and easy to control production links, the organic solvent accounts for a relatively small part of total volume of material liquid, the pollution to production equipment and environment caused by the organic solvent is reduced, and thus the method is suitable for large-scale production.
Owner:QILU PHARMA HAINAN
Method for separating and preparing liquiritin
ActiveCN101289480BHigh purityGuaranteed puritySugar derivativesPlant ingredientsMethanol waterAlcohol
The invention relates to the separation of natural medicines, in particular to a separation and preparation method of liquiritin; wherein, extracting solution is obtained by decocting the ground licorice plants with ten times of water, and then extract is obtained after the extracting solution is concentrated, and then ethanol is added for alcohol precipitation twice, and then a 6000Da molecular weight membrane separator is used for separating the extract, and then membrane separation permeation liquid is separated by X-5 macro-porous absorption resin and is eluted with the ethanol water, andthe obtained macro-porous resin separation components are separated by an efficient industrial chromatographic column and eluted with gradients with the methanol water and then the liquiritin is obtained by collecting, concentrating, freezing and drying the eluting solution. The method is high in product purity, great in preparation amount and stable and reliable in process and especially suitable for separating and preparing great amount of highly purified liquiritin compounds from the traditional Chinese medicine licorice.
Owner:浙江华谱新创科技有限公司
Preparation for effective component of turmeric
InactiveCN101317997BGuaranteed purityIncrease contentCarbonyl compound separation/purificationChromatographic separationMethanol water
The present invention provides a method for producing turmeric effective component. Turmeric material is ground and added with 70 to 95 percent of ethanol for extraction; the turmeric material is loaded on nonpolar macroporous adsorptive resin and ethanol / water is used for eluting; the eluent is collected, concentrated and dried to obtain turmeric macroporous resin component; then turmeric effective component is obtained through industrial production of chromatographic separation and mobile phase of methanol water. The main active ingredients are curcumin, demethoxycurcumin and bisdemethoxycurcumin and the total content is up to 97.3 percent. The turmeric effective component produced by the present invention gets rid of impurities and increases the content of the main active ingredients. In production, the present invention has high repeatability, good maneuverability, easy standardization and industrialization. At the same time, the present invention has certain directive significance for quality control of turmeric herb.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com