Amorphous cefodizime sodium and preparation method thereof and pharmaceutical composition comprising same
An amorphous technology of cefodizime sodium, which is applied in the field of preparation of pharmaceutical compounds, can solve problems such as impracticability, and achieve the effects of reduced irritation, low organic residual value, and very easy to satisfy in the industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
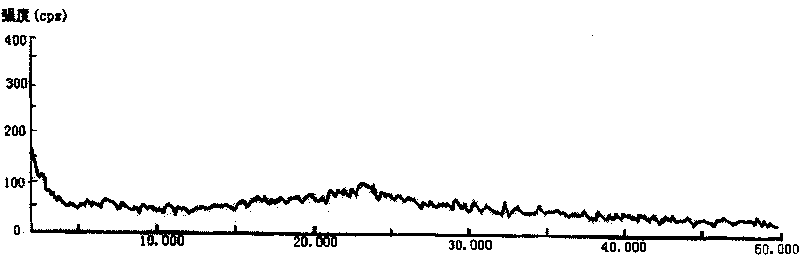
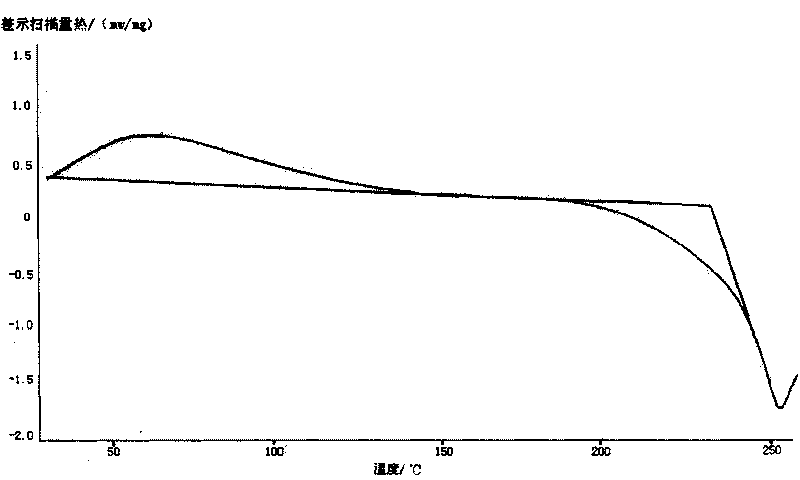
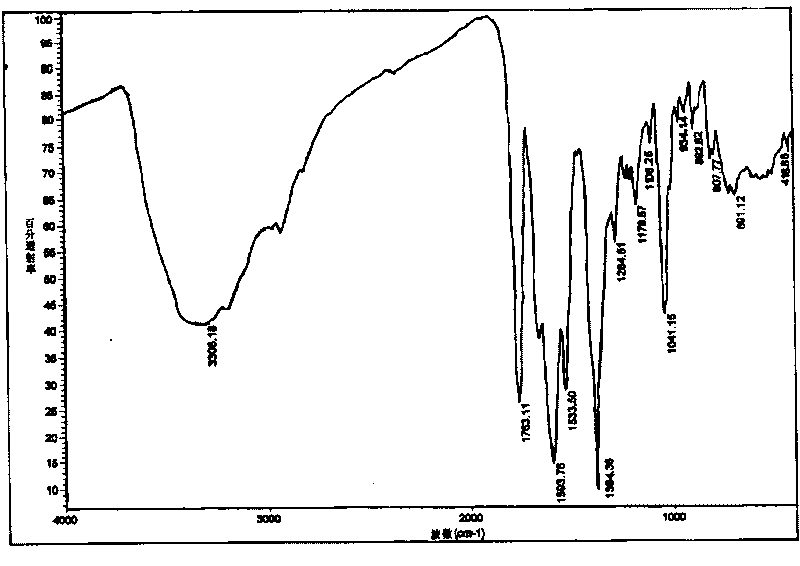
[0032] Take 5g of cefodizime sodium sample, add it to 30ml of methanol solvent, and stir at room temperature until it dissolves completely. The solution was then placed in an ice bath and cooled to about 15°C. Slowly drop 200ml of absolute ethanol into the above solution, and at the same time stir at a stirring speed of 250rpm until the solid of cefodizime sodium is completely precipitated, filter with suction, wash the filter cake with absolute ethanol and vacuum dry to constant weight to obtain 3.8g granular Amorphous cefodizime sodium product. Its X-ray diffraction pattern of gained product is as figure 1 As shown, its differential thermal analysis spectrum is shown as figure 2 As shown, its infrared spectrum is shown as image 3 shown.
[0033] The obtained granular amorphous cefodizime sodium sample and the commercially available cefodizime sodium sample are carried out to the residual ethanol test test, and the gas chromatographic analysis results show that the resi...
Embodiment 2
[0035] Take 5 g of cefodizime sodium sample, add it to 40 ml of methanol solvent, and stir at room temperature until it dissolves completely. The solution was then placed in an ice bath and cooled to about 10°C. Slowly drop 300ml of acetone into the above solution, and simultaneously stir at a stirring speed of 200rpm until the solid of cefodizime sodium is completely precipitated, filter with suction, wash the filter cake with acetone, and then vacuum-dry to constant weight to obtain 3.6g of granular amorphous cephalosporins Sodium azine products. Its X-ray diffraction collection of illustrative plates of gained product, differential thermal analysis collection of illustrative plates, infrared spectrum collection of illustrative plates are basically consistent with embodiment 1; Acetone residual content is less than 0.3%, meets the standard established by the technical guidance principle of national chemical medicine residual solvent research (acetone content should be less ...
Embodiment 3
[0037] Take 5 g of cefodizime sodium sample, add it to 25 ml of methanol solvent, and stir at room temperature until it dissolves completely. The solution was then placed in an ice bath and cooled to about 5 °C. Slowly drop 150ml of ethyl acetate into the above solution, and at the same time stir at a stirring speed of 160rpm until the solid of cefodizime sodium is completely precipitated, filter with suction, wash the filter cake with ethyl acetate and vacuum dry to constant weight to obtain 3.5g of granular Amorphous cefodizime sodium product. Its X-ray diffraction collection of illustrative plates of gained product, differential thermal analysis collection of illustrative plates, infrared spectrum collection of illustrative plates are basically consistent with embodiment 1; Ethyl acetate residual content is less than 0.3%, meets the standard that the technical guideline of national chemical drug residual solvent research makes (acetic acid The ethyl ester content should be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com