Method for preparing hydrophilic drug microsphere through spray drying process
A hydrophilic drug and spray-drying technology, which is applied in the direction of pharmaceutical formulations, organic active ingredients, medical preparations of non-active ingredients, etc., can solve the problem of low encapsulation efficiency of hydrophilic drugs, short sustained release time of drugs Difficult to produce in large quantities, etc., to overcome the large volume of oily substances and a large number of volatile solvents to wash, round the shape, and reduce the effect of organic residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
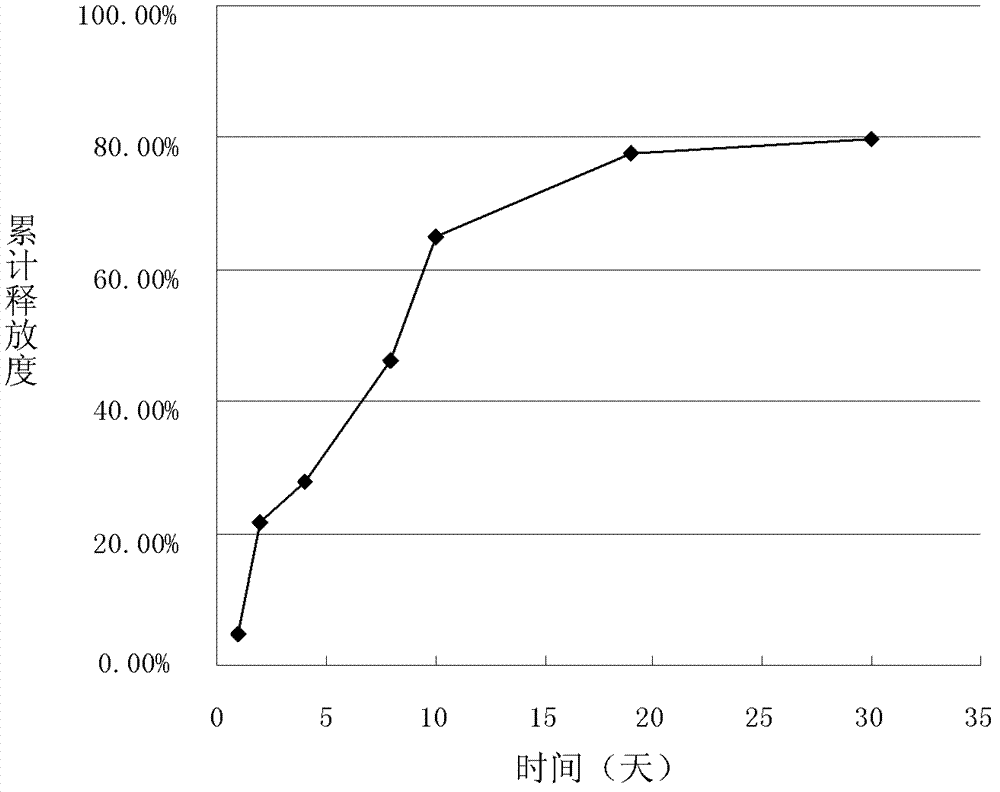
[0039]The preparation of embodiment 1 doxorubicin hydrochloride sustained-release microspheres
[0040] Raw material prescription:
[0041]
[0042]
[0043] The preparation process is as follows:
[0044] (1) Configure spray liquid
[0045] Dissolve the acrylic resin II in the ethanol solution, then add glyceryl monostearate and castor oil and stir evenly;
[0046] (2) Primary package of raw materials
[0047] Uniformly disperse doxorubicin hydrochloride in sesame oil containing Span80 to prepare S / O 1 type suspension.
[0048] (3) Homogeneous emulsification of spray liquid
[0049] Add the suspension prepared in (2) to the spray liquid obtained in (1) for homogeneous emulsification, the homogenization temperature is 50 °C, the speed of the high-speed shear homogenizer is 14000 rpm, and the time is 6 minutes to obtain S / o 1 / O 2 type spray emulsion;
[0050] (4) Spray drying: Spray dry the above-mentioned spray emulsion, the air inlet temperature is 160°C, the...
Embodiment 2
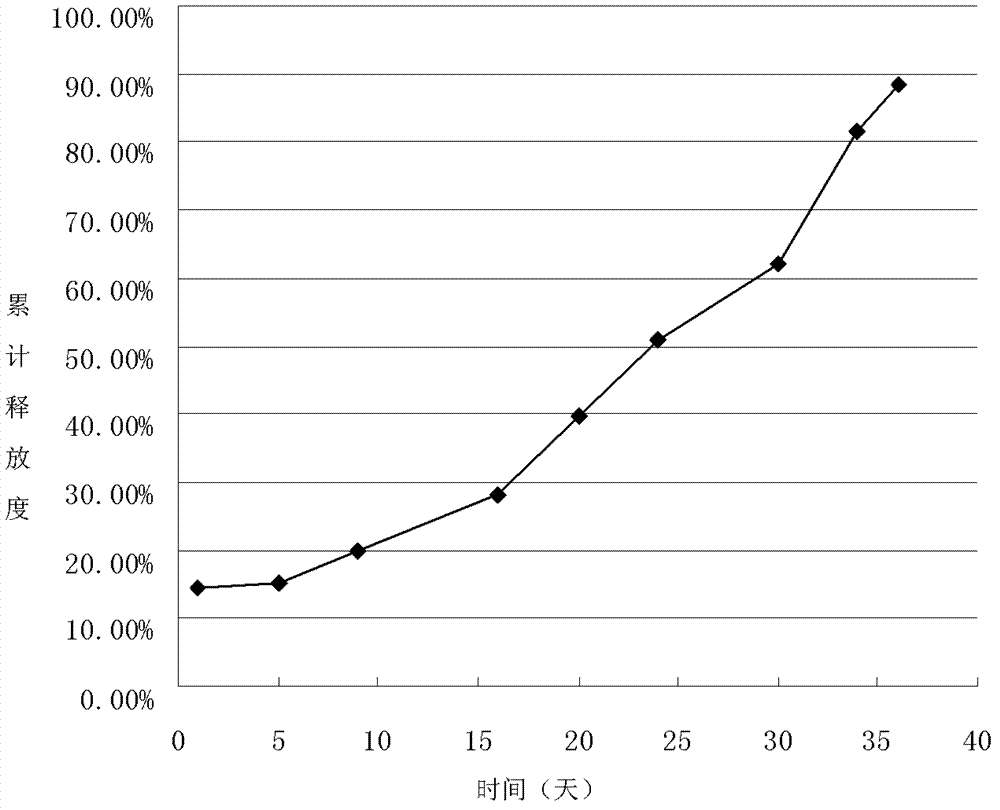
[0052] Example 2 Preparation of 5-fluorouracil sustained-release microspheres
[0053] Raw material prescription:
[0054]
[0055] The preparation process is as follows:
[0056] (1) Configure spray liquid
[0057] Dissolve acrylic resin Eudragit L100 and acrylic resin Eudragit S100 in ethanol solution, then add glyceryl monostearate and castor oil and stir well.
[0058] (2) Primary package of raw materials
[0059] Disperse 5-fluorouracil evenly in peanut oil containing Span80 to make S / O 1 type suspension.
[0060] (3) Homogeneous emulsification of spray liquid
[0061] Add the suspension prepared in (2) to the spray liquid obtained in (1) for homogeneous emulsification, the homogenization temperature is 50°C, the speed of the high-speed shear homogenizer is 10000 rpm, and the time is 9min, to obtain S / o 1 / O 2 type spray emulsion.
[0062] (4) Spray drying: Spray dry the above-mentioned spray emulsion, the air inlet temperature is 145°C, the air outlet temper...
Embodiment 3
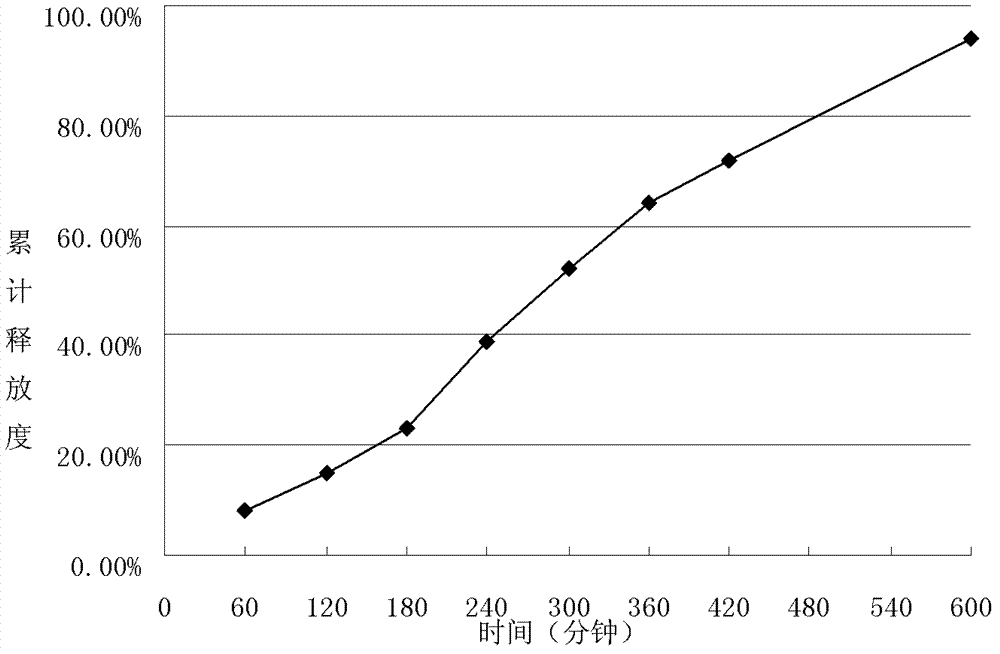
[0064] The preparation of embodiment 3 metformin hydrochloride sustained-release microspheres
[0065] Raw material prescription:
[0066]
[0067] The preparation process is as follows:
[0068] (1) Configure spray liquid
[0069] Dissolve acrylic resin Eudragit L100 in ethanol solution, then add glyceryl monostearate and castor oil and stir well.
[0070] (2) Primary package of raw materials
[0071] Disperse metformin hydrochloride evenly in sesame oil containing Span80, and prepare S / O 1 type suspension.
[0072] (3) Homogeneous emulsification of spray liquid
[0073] Add the suspension prepared in (2) to the spray liquid obtained in (1) for homogeneous emulsification, the homogenization temperature is 50°C, the speed of the high-speed shear homogenizer is 20000 rpm, and the time is 4min, to obtain S / o 1 / O 2 type spray emulsion.
[0074] (4) spray drying
[0075] The above-mentioned spray emulsion was spray-dried, the inlet air temperature was 155°C, the out...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com