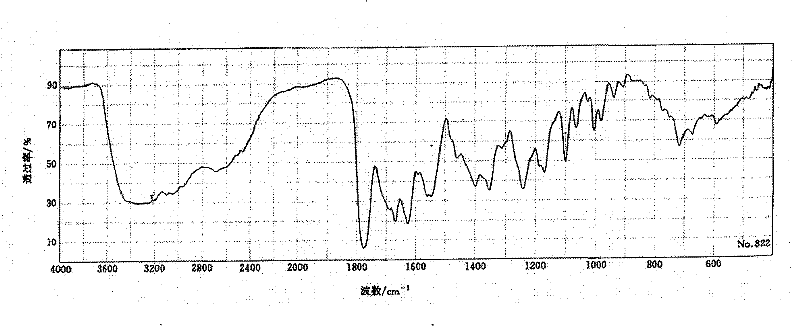
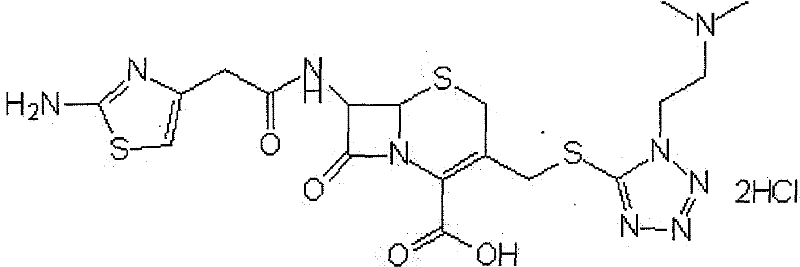
New method for purifying cefotiam hydrochloride
A kind of cefotiam hydrochloride, the technology of preparation method, applied in the field of medicine, can solve the problem of low purity of cefotiam hydrochloride and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Take 10 g of crude cefotiam hydrochloride prepared according to CN101633666B, the content of cefotiam as measured by high performance liquid chromatography is 75%, and the content of polymer as determined by gel chromatography system is 3%. The cefotiam hydrochloride crude product was dissolved in 200ml of water, treated with 20ml of 1M sodium bisulfate aqueous solution, the treatment temperature was 30°C, and the treatment time was 2 hours, then cooled to room temperature, a precipitate was precipitated, and the aqueous filtrate was obtained after filtration .
[0064] Ethyl acetate was added to the above aqueous solution to extract twice, each time the volume of the solvent used accounted for 40% of the volume of the aqueous solution, after being fully stirred, left to stand, and then the organic phase was separated to obtain an aqueous phase containing cefotiam hydrochloride.
[0065] The obtained cefotiam hydrochloride aqueous solution is raised to 45 DEG C and conc...
Embodiment 2
[0070] Get 10g cefotiam hydrochloride bulk drug (produced by Jiangsu Jiuzhitang Biological Products Co., Ltd., the year of production in August, 2010), the content of cefotiam recorded by high performance liquid chromatography is 79.5%, and the polymer content measured by gel chromatography system is 0.5%. Dissolve the crude product of cefotiam hydrochloride in 150ml of water, and treat it with 15ml of 2M potassium bisulfate aqueous solution. The treatment temperature is 40°C, and the treatment time is 1 hour. filtrate.
[0071] Cyclohexane was added to the aqueous solution for extraction three times, each time the volume of the solvent used accounted for 30% of the volume of the aqueous solution. After fully stirring, the mixture was allowed to stand, and then the organic phase was separated to obtain an aqueous phase containing cefotiam hydrochloride.
[0072] The obtained cefotiam hydrochloride aqueous solution is raised to 48 ℃ and concentrated, and concentrated to a solu...
Embodiment 3
[0075] Get the longer cefotiam hydrochloride raw material drug (Shanghai New Pioneer Pharmaceutical Co., Ltd. production, batch number 20080912) of 10g production date, the content of cefotiam recorded by high-performance liquid chromatography is 77.5%, and the gel chromatography system assay polymer The content is 2.5%. The cefotiam hydrochloride crude product was dissolved in 250ml of water, and treated with 30ml of 1M potassium bisulfate aqueous solution, the treatment temperature was 35°C, and the treatment time was 3 hours, then the temperature was cooled to room temperature, a precipitate was precipitated, and the aqueous filtrate was obtained after filtration .
[0076] Add cyclohexane and ethyl acetate 1:1 mixed solvent to the above aqueous solution and extract 3 times, the volume of the solvent used each time accounts for 25% of the volume of the aqueous solution, after fully stirring, let it stand, and then separate the organic phase to obtain An aqueous phase conta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com