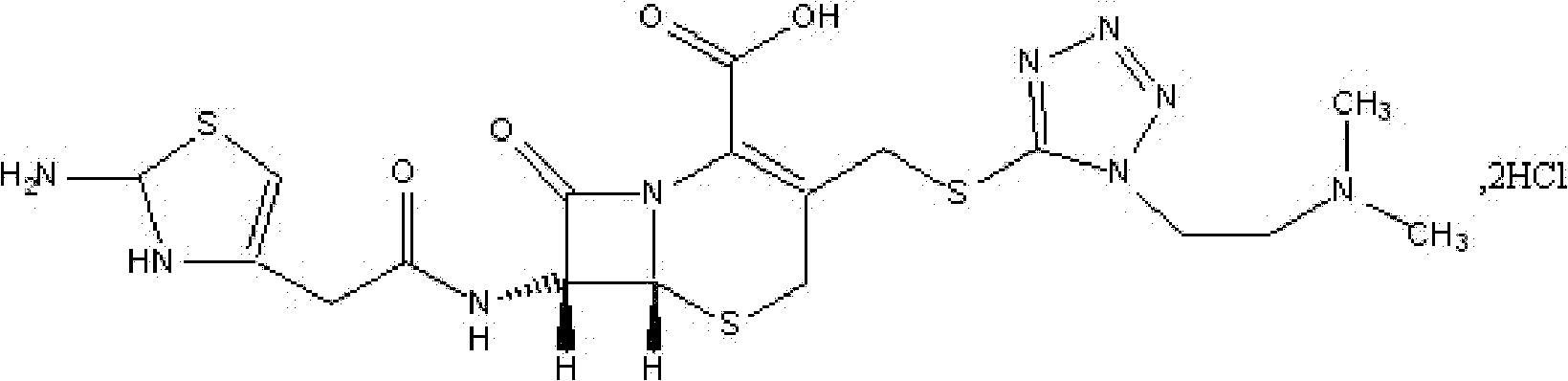
Cefotiam-hydrochloride-containing medicine preparation and preparation method thereof
A technology of cefotiam hydrochloride and pharmaceutical preparations, which is applied in the field of preparation of pharmaceutical preparations, can solve the problems of high sodium carbonate content, achieve the effects of less dosage, avoid alkalosis, and expand the scope of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 injection cefotiam hydrochloride
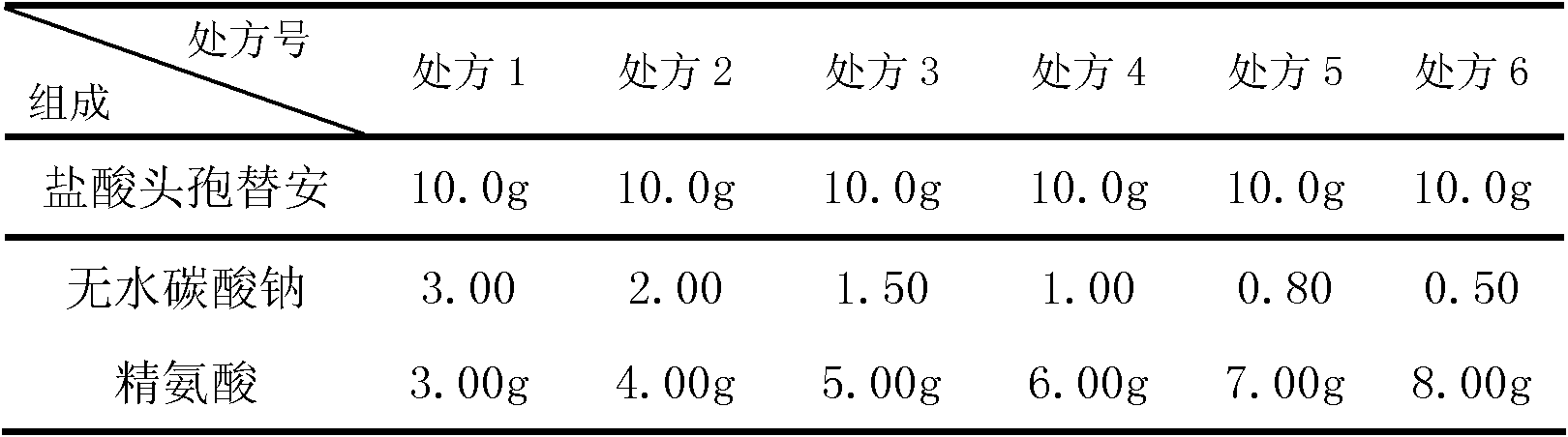
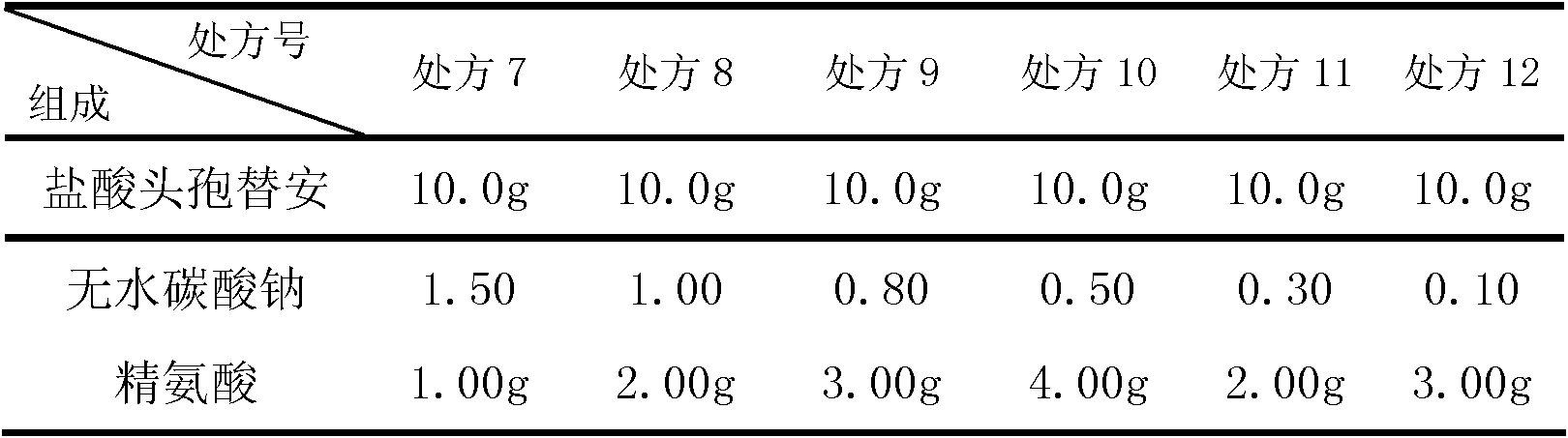
[0039] prescription:
[0040]
[0041] The preparation process is as follows:
[0042] Bottle washing: The glass bottle is first washed with ultrasonic waves, then washed with purified water, washed with water for injection, rinsed with compressed air, and finally sterilized in a tunnel oven to remove pyrogens.
[0043] Washing, drying and sterilization of rubber stoppers: wash the rubber stoppers with purified water and water for injection respectively, then steam sterilize and dry with hot air for later use.
[0044] Powder mixing: After mixing the proportioned cefotiam hydrochloride, anhydrous sodium carbonate and arginine in a mixer, samples are taken for intermediate inspection.
[0045] Packing: Aseptically pack the mixed powder into vials according to the results of the intermediate test, cap and crimp the cap.
Embodiment 2
[0046] Embodiment 2 injection cefotiam hydrochloride
[0047] prescription:
[0048]
[0049] The preparation process is as follows:
[0050]Bottle washing: The glass bottle is first washed with ultrasonic waves, then washed with purified water, washed with water for injection, rinsed with compressed air, and finally sterilized in a tunnel oven to remove pyrogens.
[0051] Washing, drying and sterilization of rubber stoppers: wash the rubber stoppers with purified water and water for injection respectively, then steam sterilize and dry with hot air for later use.
[0052] Powder mixing: After mixing the proportioned cefotiam hydrochloride, anhydrous sodium carbonate and arginine in a mixer, samples are taken for intermediate inspection.
[0053] Packing: Aseptically pack the mixed powder into vials according to the results of the intermediate test, cap and crimp the cap.
Embodiment 3
[0054] Embodiment 3 injection cefotiam hydrochloride
[0055] prescription:
[0056]
[0057] The preparation process is as follows:
[0058] Bottle washing: The glass bottle is first washed with ultrasonic waves, then washed with purified water, washed with water for injection, rinsed with compressed air, and finally sterilized in a tunnel oven to remove pyrogens.
[0059] Washing, drying and sterilization of rubber stoppers: wash the rubber stoppers with purified water and water for injection respectively, then steam sterilize and dry with hot air for later use.
[0060] Subpackaging: Aseptically subpackage the proportioned cefotiam hydrochloride, anhydrous sodium carbonate and arginine respectively into vials, cap and crimp the cap.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com