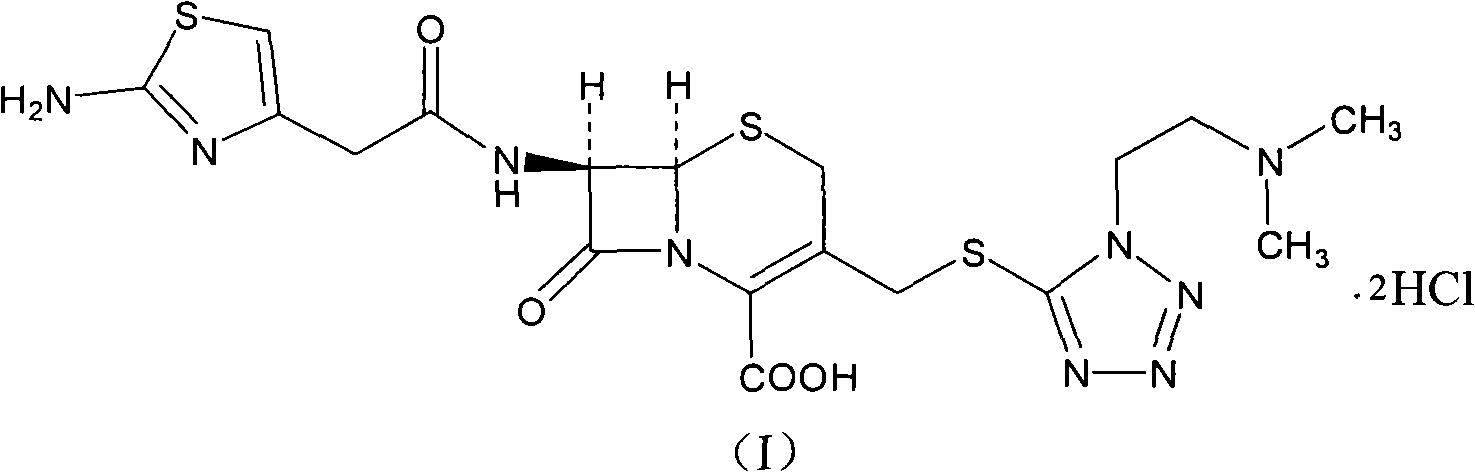
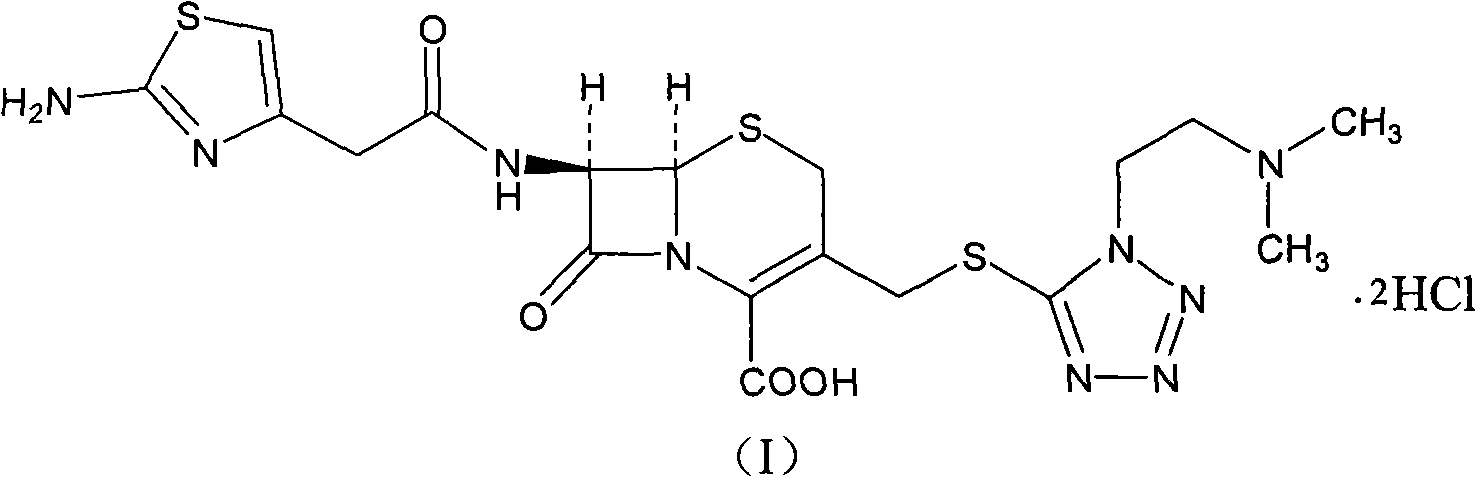
Cefotiam hydrochloride compound in new path
A technology of cefotiam hydrochloride and compounds, which is applied in the field of drug synthesis, can solve the problems of high solvent requirements, difficult operation, low yield and product purity, and achieve the effects of high product purity, reduced usage, and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
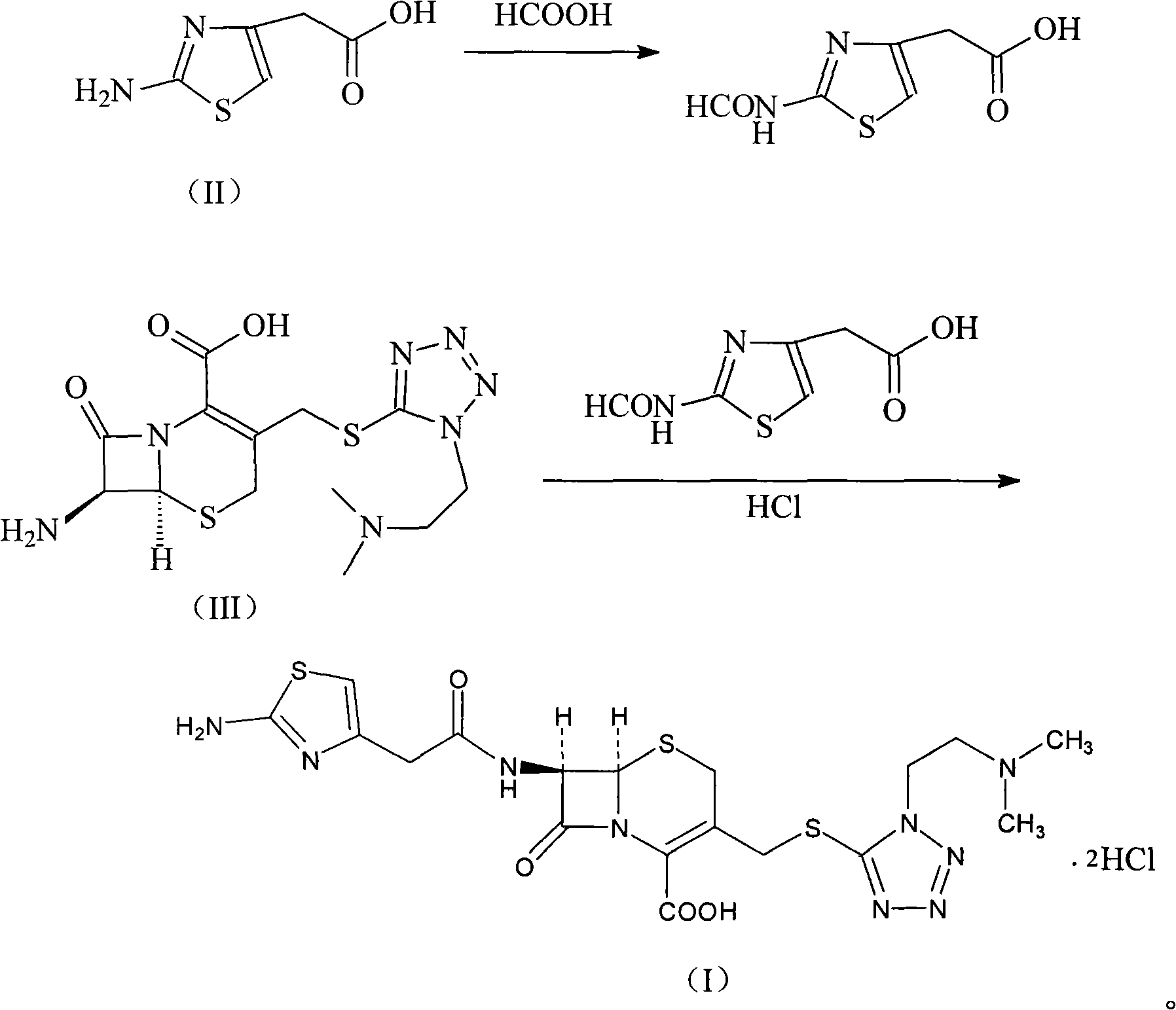
[0033] Example 1 Synthesis of 2-formylaminothiazole-4-acetic acid
[0034] In the 98% formic acid solution of 1500ml, add the 2-aminothiazole-4-acetic acid of 316 grams (2mol) and 30 grams of 4A molecular sieves, this mixture is heated to 60 ℃ of reaction 3 hours, removes unnecessary formic acid by distillation under reduced pressure, will Add 3000ml of ethyl acetate to the residue, filter to remove molecular sieves, wash the organic phase with 2000ml of distilled water, dry the organic phase with anhydrous sodium sulfate, and distill under reduced pressure to obtain 353 grams of 2-formylaminothiazole-4-acetic acid, the yield 95%.
Embodiment 2
[0035] The synthesis of embodiment 2 cefotiam hydrochloride
[0036] 93 grams of 2-formylaminothiazole-4-acetic acid and 90 ml of N, N-diisopropylethylamine are added to 400 ml of dimethylformamide, the reactant is cooled to 10 ° C, and 97 grams of ( 0.51mol) p-toluenesulfonyl chloride, stirred and reacted at this temperature for 1 hour, then added 210 grams (0.5mol) of 7-ACMT and 295ml triethylamine, stirred vigorously at 5-10°C for 0.5 hours, then added 6mol / l 320ml of hydrochloric acid was reacted at 50°C for 1 hour, then cooled to room temperature and stirred with 4 liters of acetone, the solid was precipitated, filtered, washed with acetone, and vacuum-dried at 40°C to obtain 281g of product, yield: 93.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com